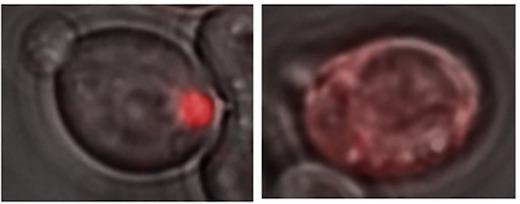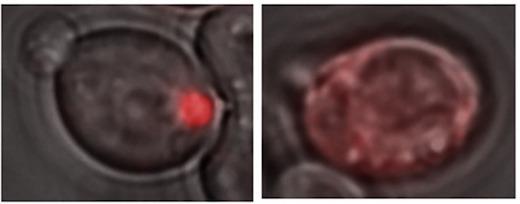
Credit: From Chernova et al Cell Reports (2017).
Prions have a notorious reputation. They cause neurodegenerative disease, namely mad cow/Creutzfeld-Jakob disease. And the way these protein particles propagate — getting other proteins to join the pile — can seem insidious.
Yet prion formation could represent a protective response to stress, research from Emory University School of Medicine and Georgia Tech suggests.
A yeast protein called Lsb2, which can trigger prion formation by other proteins, actually forms a "metastable" prion itself in response to elevated temperatures, the scientists report.
The results were published this week in Cell Reports.
Higher temperatures cause proteins to unfold; this is a major stress for yeast cells as well as animal cells, and triggers a "heat shock" response. Prion formation could be an attempt by cells to impose order upon an otherwise chaotic jumble of misfolded proteins, the scientists propose.
"What we found suggests that Lsb2 could be the regulator of a broader prion-forming response to stress," says Keith Wilkinson, PhD, professor of biochemistry at Emory University School of Medicine.
The scientists call the Lsb2 prion metastable because it is maintained in a fraction of cells after they return to normal conditions but is lost in other cells. Lsb2 is a short-lived, unstable protein, and mutations that keep it around longer increase the stability of the prions.
The Cell Reports paper was the result of collaboration between Wilkinson, Emory colleague Tatiana Chernova, PhD, assistant professor of biochemistry, and the laboratory of Yury Chernoff, PhD in Georgia Tech's School of Biology.
Other investigators studying yeast prions have been finding examples of how they may help cells adapt to a changing environment. The Emory/Georgia Tech findings are consistent with this idea, Chernova says. She notes that the Lsb2 protein's acquisition of the ability to form prions coincides in evolution with when the baker's variety of yeast (Saccharomyces), in which prion-forming Lsb2 is found, became adapted to higher temperatures.
"We don't know if these prions are adaptive," she says. "Maybe some are, while others could be pathogenic, but the whole ability of converting misfolded proteins into ordered prions instead of amorphous toxic agglomerates could be protective, and this process could be adaptive."
Understanding how and why prions form could illuminate Alzheimer's disease research; the behavior of the toxic protein fragment beta-amyloid, central in Alzheimer's, sometimes resembles that of prions. The Cell Reports authors note that the yeast protein Lsb2 has some sequence similarity to a human protein called Grb2, known to interact with APP, the precursor of amyloid-beta.
###
This research was supported by the National Institute of General Medical Sciences (GM093294) and the National Science Foundation (MCB 1516872). Investigators at St. Petersburg State University contributed to the paper.
Media Contact
Quinn Eastman
[email protected]
404-727-7829
@emoryhealthsci
http://whsc.emory.edu/home/news/index.html
############
Story Source: Materials provided by Scienmag