In a groundbreaking study, researchers from Brown University have unveiled a novel nanotechnology-driven method poised to revolutionize the treatment of fungal infections, specifically targeting the notorious Candida species, which is increasingly notorious for its drug resistance. This innovative approach revolves around the manipulation of liposomes—tiny lipid-based nanoparticles that facilitate drug delivery—bringing new hope to the field of antifungal therapy.
Fungal infections, while often overlooked, can become life-threatening particularly for vulnerable populations, such as patients undergoing chemotherapy, organ transplant recipients, and individuals in intensive care. The Candida genus, which harmlessly cohabitates the human body, can quickly morph into pathogenic forms under certain conditions, leading to severe nosocomial infections. Among its species, Candida auris has emerged as a formidable adversary due to its ability to evade current antifungal treatments, a situation that has escalated alarmingly, with reported infections surging over 300% across the United States between 2017 and 2018 alone.
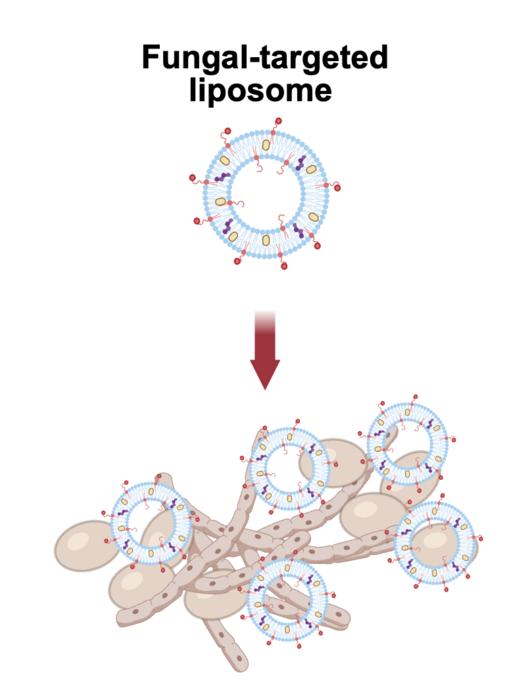
In tackling this pressing health issue, the research team at Brown has designed a specialized delivery system utilizing liposomes enhanced with targeting peptides. These peptides are short chains of amino acids chosen for their natural affinity to Candida cells, acting as a molecular GPS that guides the liposomes directly to the infection sites. Lead author Veronica LaMastro, a recent Ph.D. graduate in biomedical engineering, explained that this targeted delivery strategy significantly boosts both the specificity and effectiveness of antifungal agents, thus holding great promise against resistant strains.
The anatomical structure of these liposomes—spherical aggregates made from synthetic and organic lipids—enables them to encapsulate therapeutic agents within their lipid bilayers. By strategically decorating the liposomal surface with the peptide known as penetratin, the researchers have succeeded in amplifying their liposome’s binding affinity for Candida cells. This meticulous design resulted in notably improved interaction rates with the pathogens compared to conventional, non-targeted liposomes, confirming the potential of this targeting approach.
Evidence from extensive lab tests revealed compelling findings: the peptide-decorated liposomes not only excelled in targeting Candida cells but also delivered the ant fungal agent posaconazole with remarkable efficacy. This FDA-approved drug, traditionally utilized as a prophylactic against Candida overgrowth, when paired with the targeted liposomal system, achieved inhibitory concentrations up to eight times lower than previously required. Astonishingly, it demonstrated the ability to prevent biofilm formation at doses up to 1,300 times more effective than free posaconazole alone.
In the clinical context, Candida biofilms represent a significant challenge in treating infections, as these robust structures are notoriously resilient against conventional antifungal therapies and enable Candida to persist and propagate. By employing liposomes that deliver concentrated antifungal doses directly to biofilm sites, the research team posits substantial advancements in clinical treatment protocols.
To validate the therapeutic potential of their targeted liposomes in a living system, the team employed a mouse model of intradermal Candida albicans infections. Their findings underscored the promising utility of this novel delivery platform: mice treated with targeted liposomes exhibited a staggering 60% reduction in fungal burden compared to those receiving standard drug-loaded liposomes. These results illuminate a vital pathway forward in combatting the escalating threat posed by drug-resistant fungal pathogens.
As antifungal drug resistance continues to plague clinical medicine, the work spearheaded by Professor Anita Shukla and her colleagues at Brown’s School of Engineering emerges as a timely contribution to the field of biomedical engineering. Shukla emphasizes the critical need for innovation in the area of fungal research, especially given the rising tide of antimicrobial resistance.
This pioneering study not only provides insights into the mechanisms of enhanced antifungal targeting but also advocates for a broader exploration of similar targeted nanotechnology approaches across different infectious agents. The implications of these findings could reshape strategies in antifungal treatment, moving from conventional methodologies to cutting-edge, precision-targeted therapeutics.
Moreover, the research team is motivated to further refine and expand their platform. While their current study focused primarily on preventive measures using posaconazole, future investigations aim to adapt this innovative liposomal delivery mechanism for treating established fungal infections, potentially addressing a vast array of clinical scenarios.
The successful interplay between biotechnology and therapeutic application showcased in this study heralds a new era of antifungal innovation, underscoring the importance of specialized targeting in achieving effective treatment outcomes against resilient and often deadly fungal infections. Through continued examination of this methodology, researchers hope to further illuminate the pathways toward enhanced patient care and health outcomes in vulnerable populations grappling with the burden of opportunistic fungal infections.
These exciting developments signify a crucial pivot in our understanding of and approach to managing fungal infections, reinforcing the notion that innovative technology can provide solutions to longstanding medical challenges and improve the overall effectiveness of clinical interventions.
With the continued threat of Candida species exhibiting resistance to established treatments, this targeted peptide-decorated liposomal technology offers new avenues for discovery and application, urging a concerted effort from the scientific community to prioritize research in this vital field.
The study was funded by the National Science Foundation, highlighting the significance of supporting pioneering research aimed at addressing critical healthcare challenges. Moving forward, the research team remains committed to expanding the application of their novel technology, advocating for recognition of the pressing need to combat fungal infections with renewed vigor and innovation.
In summary, the emergence of this targeted liposomal system manifests a beacon of hope in the fight against fungal infections, paving the way for future advancements that can enhance the arsenal of treatments available to healthcare professionals facing mechanical resistance in clinical settings.
Subject of Research: Animals
Article Title: Peptide-Decorated Liposomes Enhance Fungal Targeting and Antifungal Drug Delivery
News Publication Date: 9-May-2025
Web References: Advanced Functional Materials
References: N/A
Image Credits: Credit: Shukla Lab / Brown University
Keywords
Fungal infections, Candida, liposomes, drug delivery, antimicrobial resistance, biofilms, nanotechnology, biomedical engineering, posaconazole.
Tags: antifungal treatmentsBrown University researchCandida auris challengesCandida speciesdrug-resistant fungal infectionsfungal infection therapyliposome technologyliposomes and peptidesnosocomial infectionsnovel drug delivery systemstargeted nanoparticlesvulnerable patient populations