In organic chemistry, π-stacking systems are supramolecular structures that arise due to the dispersion force, a type of intermolecular noncovalent interaction. They are a common occurrence in nature; the stabilized structure of DNA is a very prominent example of a π-stacking system, and so are the arrangement of amino acids in certain proteins. Interestingly, π-stacking can be leveraged in the design of materials with useful electronic and optical properties. These include organic semiconductors of various kinds, as well as conjugated polymers for sensing and biomedical applications.
Credit: Hiromitsu Maeda from Ritsumeikan University, Japan
In organic chemistry, π-stacking systems are supramolecular structures that arise due to the dispersion force, a type of intermolecular noncovalent interaction. They are a common occurrence in nature; the stabilized structure of DNA is a very prominent example of a π-stacking system, and so are the arrangement of amino acids in certain proteins. Interestingly, π-stacking can be leveraged in the design of materials with useful electronic and optical properties. These include organic semiconductors of various kinds, as well as conjugated polymers for sensing and biomedical applications.
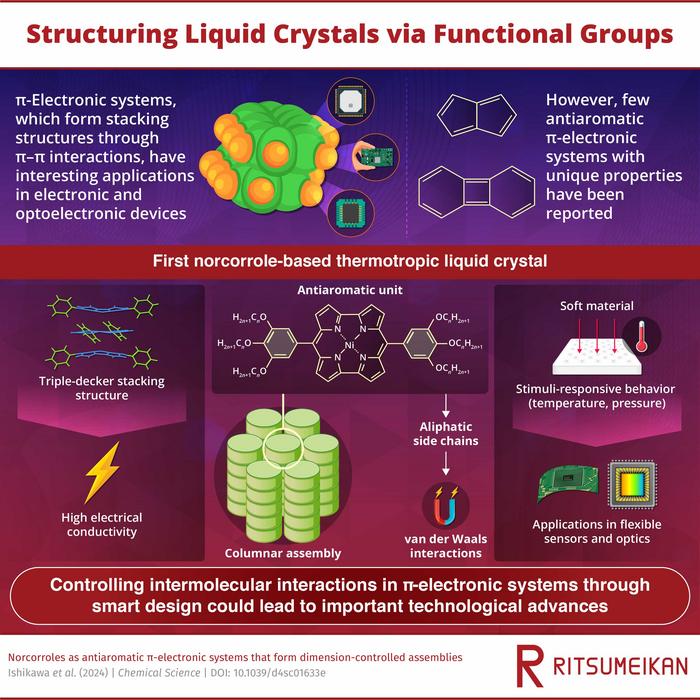
Thus far, a good portion of technologically relevant π-stacking system has been limited to aromatic compounds, which have inherent π-electron clouds. On the other hand, antiaromatic compounds, though promising candidates for developing electric conductors, have been scarcely reported as the building units of π-stacking systems.
Surprisingly, in a recent study, a research team led by Professor Hiromitsu Maeda from Ritsumeikan University, Japan, reported a novel antiaromatic π-stacking system that enabled the formation of a highly conductive liquid crystal. Their findings were published on April 16, 2024, in the journal Chemical Science. Worth noting, this paper was co-authored by Prof. Go Watanabe from Kitasato University, Prof. Shu Seki from Kyoto University, and Prof. Hiroshi Shinokubo from Nagoya University.
The reported compounds in question are NiII-coordinated norcorroles with modified aryl moieties as side chains. Previously, achieving π-stacking in similar norcorroles failed because hydrogen-bonding interactions between the side chains opposed the face-to-face stacking of the planar antiaromatic units. This time, however, the research team had an ingenious idea. “We hypothesized that the introduction of side interacting moieties with less directionality would enhance the stacking between norcorrole units,” explains Prof. Maeda. “Thus, we attempted the simple introduction of aliphatic chains, which induce van der Waals interactions. These interactions can be effective for modulating the stacking structure of a material,” adds Prof. Maeda.
As evidenced through various experiments and molecular dynamics simulations, the proposed strategy worked as intended. The norcorrole units formed columnar structures through the stacking of arrangements known as ‘triple-decker.’ In these arrangements, a planarized molecule is sandwiched between two slightly bowl-shaped molecules.
Using the proposed molecular design, the researchers then synthesized liquid crystals. Thanks to the triple-decker stacking, a liquid crystal exhibited remarkable electric conductivity as well as thermotropicity; that is, an order parameter that depends on temperature. “The control of molecular interactions based on molecular design and synthesis, as demonstrated in our study, will be crucial for future applications,” remarks Prof. Maeda. “Properties such as high electric conductivity in liquid crystals may be used for the fabrication of electronic devices. In addition, stimuli-responsive behaviors in soft materials can be used to modulate relevant properties, like photoluminescence, according to pressure and temperature,” explains Prof. Maeda.
Taken together, the findings of this study bring to light a promising strategy for designing new compounds based on molecular assemblies of antiaromatic units. With any luck, this will open up new avenues for materials design, ultimately leading to better organic electronics, optoelectronics, and sensing devices.
***
Reference
DOI: https://doi.org/10.1039/D4SC01633E
About Ritsumeikan University, Japan
Ritsumeikan University is one of the most prestigious private universities in Japan. Its main campus is in Kyoto, where inspiring settings await researchers. With an unwavering objective to generate social symbiotic values and emergent talents, it aims to emerge as a next-generation research university. It will enhance researcher potential by providing support best suited to the needs of young and leading researchers, according to their career stage. Ritsumeikan University also endeavors to build a global research network as a “knowledge node” and disseminate achievements internationally, thereby contributing to the resolution of social/humanistic issues through interdisciplinary research and social implementation.
Website: http://en.ritsumei.ac.jp/
Ritsumeikan University Research Report: https://www.ritsumei.ac.jp/research/radiant/eng/
About Professor Hiromitsu Maeda from Ritsumeikan University, Japan
Dr. Hiromitsu Maeda is a professor at the Department of Applied Chemistry, College of Life Sciences, Ritsumeikan University and a Ritsumeikan Advanced Research Academy (RARA) Fellow. He completed his PhD from Kyoto University in 2004. Professor Maeda’s research interests include topics like physical organic chemistry, supramolecular chemistry, and materials science on π-electronic systems. Prof. Maeda has received several prizes, including the ChemComm Emerging Investigator Lectureship (2012) and Fellow of the RSC (2015), and has over 200 publications.
Funding information
This work was supported by JSPS KAKENHI Grant Numbers JP18H01968, JP22H02067, and JP23K23335 for Scientific Research (B), JP19K05444 and JP2408389 for Scientific Research (C), JP23K17951 for Challenging Research (Exploratory), JP20H05862, JP20H05863, and JP20H05867 for Transformative Research Areas (A) “Condensed Conjugation”, JP19H05718 for Scientific Research on Innovative Areas “Aquatic Functional Materials”, Ritsumeikan Global Innovation Research Organization (R-GIRO) project (2017–22 and 2022–27), JST SPRING Grant Number JPMJSP2101, JST CREST Grant Number JPMJCR23O1, and the Sasakawa Scientific Research Grant from the Japan Science Society.
Journal
Chemical Science
DOI
10.1039/D4SC01633E
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Norcorroles as antiaromatic π-electronic systems that form dimension-controlled assemblies
Article Publication Date
16-Apr-2024
COI Statement
There are no conflicts of interest to declare.




