Fukuoka, Japan—Solid oxide fuel cells, or SOFC, are a type of electrochemical device that generates electricity using hydrogen as fuel, with the only ‘waste’ product being water. Naturally, as we strive to reduce our carbon output and mitigate the casualties of the climate crisis, both business and academia have taken major interest in the development of SOFCs.
Credit: Kyushu University/Yamazaki Lab
Fukuoka, Japan—Solid oxide fuel cells, or SOFC, are a type of electrochemical device that generates electricity using hydrogen as fuel, with the only ‘waste’ product being water. Naturally, as we strive to reduce our carbon output and mitigate the casualties of the climate crisis, both business and academia have taken major interest in the development of SOFCs.
In what can potentially accelerate the development of more efficient SOFCs, a research team led by Kyushu University has uncovered the chemical innerworkings of a perovskite-based electrolyte they developed for SOFCs. The team combined synchrotron radiation analysis, large-scale simulations, machine learning, and thermogravimetric analysis, to uncover the active site of where hydrogen atoms are introduced within the perovskite lattice in its process to produce energy. The results were published in journal Chemistry of Materials.
At the fundamental level, a fuel cell is just a device that generates electricity by facilitating the split of a hydrogen atom into its positively charged proton and negatively charged electron. The electron is used to generate electricity, and then comes together with a proton and oxygen and produces water as a ‘waste’ product.
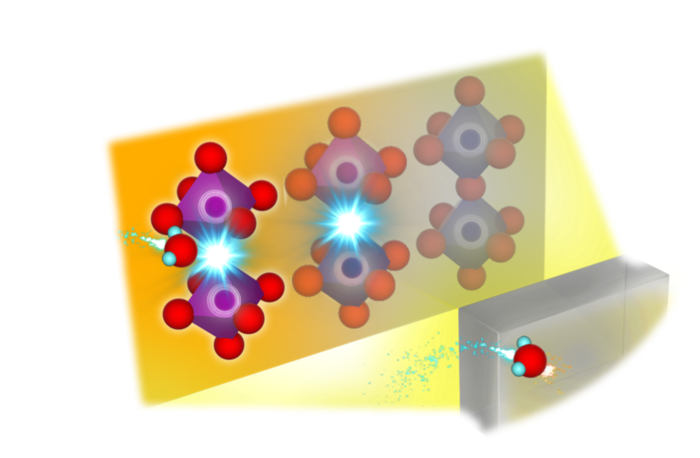
The material at the literal center of all this is the electrolyte. This material acts an atomic sieve that facilitates transfer of specific atoms across the fuel cell. Depending on the type of fuel cell, those atoms could be protons or oxygen.
While SOFCs may be an uncommon term to many people, the technology has already been commercialized in generators for single family homes. Nonetheless, they remain expensive, with one of the largest obstacles being its high operating temperature.
“Conventional SOFCs need to be at 700-1000℃ for the electrolyte to perform efficiently.” explains Professor Yoshihiro Yamazaki at Kyushu University’s Platform of Inter-/Transdisciplinary Energy Research, who led the research. “Naturally, there’s a global race to develop SOFC electrolytes that can operate at lower temperatures of around 300-450℃. One such promising materials are perovskites.”
Perovskites are a category of material with a specific crystalline structure that allows them to possess unique physical, optical, and even electrical properties. Moreover, since they can be artificially synthesized with different atoms, a large body of research focuses on developing and testing a near infinite number of possible perovskites.
One such case is in developing better SOFC electrolytes.
“In our past work we developed a Barium and Zirconium based perovskite with the chemical composition BaZrO3. By replacing the Zr site with a high concentration of Scandium, or Sc, we succeeded in making a high-performance electrolyte that can function at our target temperature of 400℃,” explains Yamazaki. “Of course, that was only a part of what we wanted to find. We also were investigating a question that hadn’t been solved for over three decades: where in the electrolyte’s lattice do the protons get introduced?”
Probing the inner workings of SOFCs had been difficult due to its high operating temperature and changing pressure from water, the fuel cell’s source of hydrogen.
To get around these issues, the team conducted X-ray absorption spectroscopy experiments on their perovskite electrolyte using synchrotron radiation—the electromagnetic radiation emitted from particle accelerators—while the fuel cell was active at around 400℃.
“These results gave us insight into where in the material’s chemical structure the protons would be incorporated. From there we applied machine learning, and using a supercomputer calculated possible structural configurations of the material,” continued Yamazaki. “By carefully comparing the predicted results with experimental data we were able to clarify the structural changes the electrolyte undertakes when active.”
“Now that we have the fundamental innerworkings of the electrolyte we can being optimizing its nanostructures and even propose new materials that can lead to more efficient fuel cells, and even ones that work at wider temperature ranges,” concludes Yamazaki.
###
For more information about this research, see “Probing Local Environments of Oxygen Vacancies Responsible for Hydration in Sc-Doped Barium Zirconates at Elevated Temperatures: In Situ X-ray Absorption Spectroscopy, Thermogravimetry, and Active Learning Ab Initio Replica Exchange Monte Carlo Simulations,” Kenta Hoshino, Shusuke Kasamatsu, Junji Hyodo, Kentaro Yamamoto, Hiroyuki Setoyama, Toshihiro Okajima, and Yoshihiro Yamazaki, Chemistry of Materials, https://doi.org/10.1021/acs.chemmater.2c02116
About Kyushu University
Kyushu University is one of Japan’s leading research-oriented institutes of higher education since its founding in 1911. Home to around 19,000 students and 8,000 faculty and staff, Kyushu U’s world-class research centers cover a wide range of study areas and research fields, from the humanities and arts to engineering and medical sciences. Its multiple campuses—including one of the largest in Japan—are located around Fukuoka City, a coastal metropolis on the southwestern Japanese island of Kyushu that is frequently ranked among the world’s most livable cities and historically known as Japan’s gateway to Asia. Through its Vision 2030, Kyushu U will ‘Drive Social Change with Integrative Knowledge.’ Its synergistic application of knowledge will encompass all of academia and solve issues in society while innovating new systems for a better future.
Journal
Chemistry of Materials
DOI
10.1021/acs.chemmater.2c02116
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Probing Local Environments of Oxygen Vacancies Responsible for Hydration in Sc-Doped Barium Zirconates at Elevated Temperatures: In Situ X-ray Absorption Spectroscopy, Thermogravimetry, and Active Learning Ab Initio Replica Exchange Monte Carlo Simulations
Article Publication Date
14-Mar-2023



