Princeton Chemistry’s Scholes Group is reporting evidence that quantum vibrations participate in electron transfer
Credit: Image courtesy of Bo Fu, Princeton Chemistry.
Princeton Chemistry’s Scholes Group is reporting evidence that quantum vibrations participate in electron transfer, establishing with ultrafast laser spectroscopy that the vibrations provide channels through which the reaction takes place.
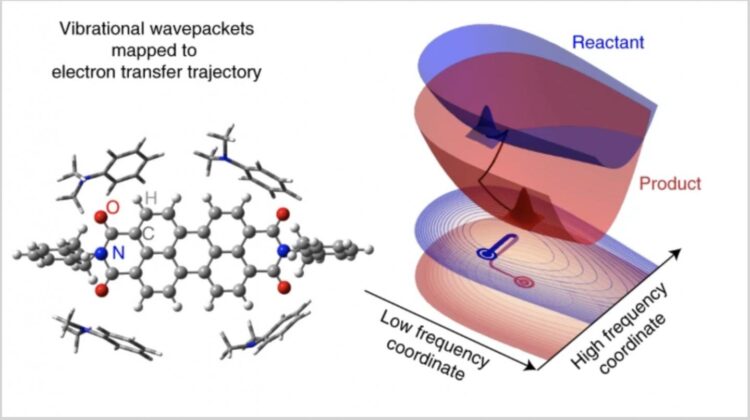
Seeking to establish an experimental proof for a highly contested topic – the role of vibrations in processes fundamental to solar energy conversion – Princeton researchers set out to map the progress of a photoinduced electron transfer (ET) reaction.
The short laser pulses in ultrafast spectroscopy helped to lock all the light-absorbing entities in-step. Researchers were then able to watch the electron transfer dynamics and the vibrational dynamics simultaneously through beats created by the vibrational coherences. They found that the photoinduced ET reaction occurs in ~30-femtoseconds, which contrasts with conventional Marcus theory, and concluded that the unexpectedly rapid pace of the reaction revealed some unknown mechanisms at play.
“What we found is a unique cascade of quantum mechanical events occurring succinctly with the electron transfer reaction,” said Shahnawaz Rafiq, a former postdoc in the Scholes Group and lead author of the paper. “These events appear sequentially in the form of loss of phase coherence along high-frequency vibrations, followed by impulsive appearance of a phase coherence along a low-frequency vibration.
“These two events of quantum nature occur because of the role these vibrations play in enabling this ET reaction,” said Rafiq. “That’s a major part of what we’re reporting: how we’re able to pinpoint certain places in spectral data that tell us, oh, this is the point of importance. It’s a needle in a haystack.”
In addition, researchers found an extra vibrational wavepacket in the product state, which was not there in the reactant state.
“It is as if the ET reaction itself created that wavepacket,” said Rafiq. “The ultimate revelation is that there is an order to the structural changes associated with a reaction that is decided by the frequencies of the vibrational modes.”
The paper, “Interplay of vibrational wavepackets during an ultrafast electron transfer reaction,” was published this week online in Nature Chemistry. It marks the culmination of two years of work.
The challenge researchers set themselves in this investigation involved parsing out vibrational coherences relevant to the ET reaction from the vast number of coherences generated by the laser excitation, most of which are spectators.
In their data, researchers discovered the abrupt loss of phase coherence along some high-frequency vibrational coordinates. This rapid loss of phase coherence originates from the random phase interference of ET reaction pathways provided by the vibrational ladder. The observation steps beyond the conventional Marcus theory and directly reports on the vibrationally driven reaction trajectory from the reactant state to the transition state.
“We create wavepackets on the reactant state by using laser pulses, and these wavepackets start dephasing irreversibly from then on,” said Rafiq. “So, we do not anticipate seeing any extra wavepacket in the product state. We can see some of them dephase abruptly because they participate in the reaction, but then, seeing a new wavepacket appearing on the product state was tantalizing.”
Bo Fu, a postdoc in the Scholes Group and co-author of the paper, added, “Researchers always think that the wavepacket can only be generated by a photon pulse. But here we observe a wavepacket that did not seem to be generated by the photon pulse. Seeing it on the product state indicates a different mechanism of its generation. Quantum dynamics simulations helped us establish that this wavepacket was actually generated by the ET reaction.”
Researchers likened the wavepacket generation by ET to stretching a vibrating spring to a more stable position, with an added property that the spring vibrates with a significantly larger amplitude about its new mean position. This spring-like response of the synchronized beating of the molecular structure to the ET provides a sink that inhibits coherent recurrence of the ET, which might otherwise be expected for a process that occurs vectorially than stochastically.
“What I like about this work is that it shows how the structure of a molecular complex distorts during a reaction,” said Gregory Scholes, the William S. Tod Professor of Chemistry and a co-author on the paper. “And this distortion happens as a logical sequence of events–just like the molecules were made of springs. The stiff springs respond first, the soft springs last.”
The Scholes Group is interested in ultrafast processes in chemistry, seeking to answer questions about energy transfer, excited state processes, and what happens after light is absorbed by molecules. These questions are addressed both theoretically and experimentally.
###
Media Contact
Wendy Plump
[email protected]
Original Source
http://www.