FOR IMMEDIATE RELEASE
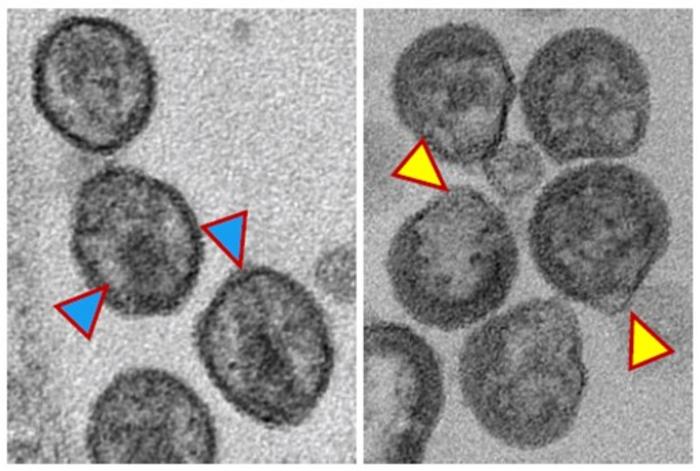
Credit: Image provided by Abdul Waheed, Ph.D., NIH
FOR IMMEDIATE RELEASE
Researchers at Johns Hopkins Medicine, in collaboration with researchers at the National Institutes of Health, report that two new studies in mice with a humanized immune system and human cell lines have identified an enzyme that plays a critical role in the late stages of HIV replication.
This enzyme, called neutral sphingomyelinase-2 (nSMase2), plays an important role in the body’s metabolism of lipids, types of naturally occurring fats found in cells. When nSMase2 was blocked or deleted, the HIV produced was oddly shaped, did not mature and was not infectious. Eventually, inhibiting nSMase2 in HIV-infected cells resulted in the death of the infected cell. A new class of antiretroviral compounds developed at Johns Hopkins that block nSMase2 are promising to advance the potential for treating HIV infections by not only suppressing HIV replication, but also by killing the cells that are infected.
The findings were laid out in a pair of studies published July 5 in the journal Proceedings of the National Academy of Sciences.
HIV-1 is the most common type of HIV that attacks the immune system and leads to AIDS. The virus takes control and destroys certain white blood cells, including CD4+ T-cells. There is no cure for HIV, but antiretroviral medications can suppress the replication of the virus and prevent progression to AIDS. However, if people stop taking their medications, the virus starts replicating again. Because the virus needs a protective covering to infect other cells, these studies focused new attention on the biology and activity that creates this “viral envelope.”
“The HIV genome is very small, so to replicate itself, it hijacks all sorts of cellular machinery. Neutral sphingomyelinase-2 is one piece of this hijacked machinery that we found is absolutely essential for HIV to properly replicate itself,” says Norman Haughey, Ph.D., professor of neurology at the Johns Hopkins University School of Medicine and a co-corresponding author of both studies.
For the first of the newly reported studies, researchers built on earlier observations of the role of the enzyme nSMase2. This cellular enzyme breaks down complex fats in cell membranes into simpler forms called ceramides. This process helps create a platform on which new virus particles can form. When the virus leaves the cell, it takes a part of the cell’s membrane as its own covering. Ceramide, a specific type of fat in this covering, seems to be crucial for the final stages of the virus’s reproduction.
“We show in these papers that if we target the enzyme nSMase2 in a cell that’s making the virus, it changes the lipid composition of the virus particle, and this blocks virus maturation and thus virus infectivity,” says Eric Freed, Ph.D., a co-corresponding author of both studies and director of the HIV Dynamics and Replication Program at the Center for Cancer Research at the National Cancer Institute within the National Institutes of Health.
The second study tested an nSMase2-inhibiting experimental compound called PDDC on the mice with human-like immune systems that were infected with HIV. The compound reduced HIV levels in the blood to undetectable levels, similar to the current treatment used by people with HIV. When the regular treatment was stopped, HIV returned quickly. However, with PDDC, 80% of the treated animals didn’t have a rebound in HIV. Haughey says the compound seems to target and kill the cells that HIV needs to reproduce, stopping the virus from infecting other cells.
“Even after four decades of research, there are still parts of the HIV replication cycle we do not understand. These studies add critical knowledge of how HIV replicates,” says Freed. Haughey adds that their results offer the first demonstration of using an nSMase2 blocker to stop actively replicating HIV in living cells, and the first antiretroviral target that is capable of killing infected cells. “We’re talking about a new target that has the potential for a new therapeutic approach for treating HIV infection,” Haughey says.
“We still have a long way to go, this is just a piece of the puzzle. This enzyme is one foot in the door to better understanding the viral lipid composition in the late stages of the replication cycle,” Freed cautioned, as many more preclinical studies must be done before any attempt is made to use nSMase2 inhibitors in people infected with HIV.
Other researchers include Seung-Wan Yoo, Abdul Waheed, Yanan Zhu, Eva Agostino, Lwar Naing, Yuta Hikichi, Ferri Soheilian, Yun Song, Peijun Zhang, Barbara Slusher, Pragney Deme, Seuhmus Tohumeken, Rana Rais, Matt Smith, Catherine DeMarino, Peter Calabresei and Fatah Kashanchi.
These studies were funded by grants from the National Institute of Mental Health (R01MH131469 and P30MH075673) and the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, both part of the National Institutes of Health.
Haughey and Slusher are listed in patent applications filed by Johns Hopkins Technology Ventures covering novel compositions and utilities of nSMase2 inhibitors, including PDDC. This arrangement has been reviewed and approved by The Johns Hopkins University in accordance with its conflict-of-interest policies.
Journal
Proceedings of the National Academy of Sciences