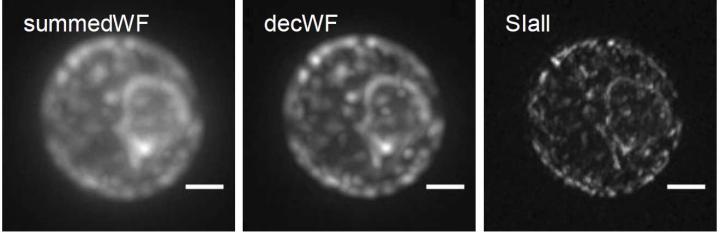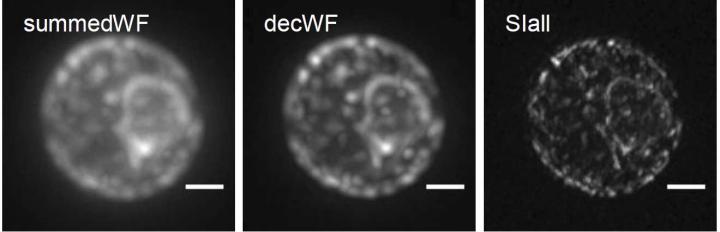
Credit: Stelzer Research Group, Goethe University Frankfurt
FRANKFURT. Is it possible to watch at the level of single cells how fish embryos become trout, carp or salmon? Researchers at Goethe University Frankfurt have successfully combined two very advanced fluorescence microscopy techniques. The new high-resolution light microscope permits fascinating insights into a cell's interior.
Using the "light-sheet microscopy" technology invented and developed by Professor Ernst Stelzer, it was already possible to observe organisms in a very precise and vivid way during cell differentiation. His group at Goethe University Frankfurt has now combined light sheets with a technique which so far only allowed very high spatial resolutions (Light-sheet-based fluorescence microscopy (LSFM) is the most recent three-dimensional fluorescence microscopy technique. In fluorescence microscopy, a fraction of a cell's molecules is labelled with fluorescent markers, which are lit up with a beam of light. A camera records the three-dimensional distribution of the fluorescing molecules, i.e. the fluorophores. The outstanding advantage of LSFM is that even sensitive samples such as fish embryos survive observation. This is a major advancement since conventional methods, which illuminate the whole sample, expose the specimens to much more energy and destroy the cells in a very short period of time.
Ernst Stelzer, professor at the Institute of Cell Biology and Neuroscience and a principal investigator in the Cluster of Excellence "Macromolecular Complexes" of Goethe University Frankfurt, explains that LSFM does not illuminate the entire sample but only micrometre-thin light sheets. "Since we examine the biological specimens under conditions that are as natural as possible, we achieve very precise results", says Stelzer. However, not only static images of cells but also dynamic changes in their environment or genetic mutations can be measured in direct comparisons.
Bo-Jui Chang, Victor Perez Meza and Ernst Stelzer have now improved the technique further: "We combined light-sheet fluorescence microscopy with coherent structured illumination microscopy (SIM). This allows for an extremely high resolution", he reports. SIM is a super-resolution technique that produces several images, which are combined digitally. As a result, resolution is improved in the physical sense. The technical approach is to excite a fluorescing sample with a very specific illumination pattern. Sub-100 nm resolutions with this method are limited to surfaces but the technique has major advantages. It is fairly moderate in the excitation of the fluorescence, allows very fast imaging and can be used with all fluorescing molecules for high-resolution purposes.
"In the new microscope, which we call csiLSFM, we have developed the principle of SIM further in such a way that sub-100 nm resolutions are no longer limited to surfaces but can also be used in extensive three-dimensional objects. Here, two counterpropagating light sheets interfere at an angle of 180° so that they form the smallest possible linear interference pattern. As a result, we achieve an optimal resolution of less than 100 nanometres," explains Ernst Stelzer. The new instrument has three objective lenses. It works via the flexible control of rotation, frequency and phase shift of the perfectly modulated light sheet.
Images of endoplasmic reticulum of yeast, a complex membrane network of tubules, vesicles and cisterns, show that the researchers can use csiLSFM to work successfully with physiologically important objects.
###
Media Contact
Dr. Ernst H. K. Stelzer
[email protected]
@goetheuni
http://www.uni-frankfurt.de
############
Story Source: Materials provided by Scienmag