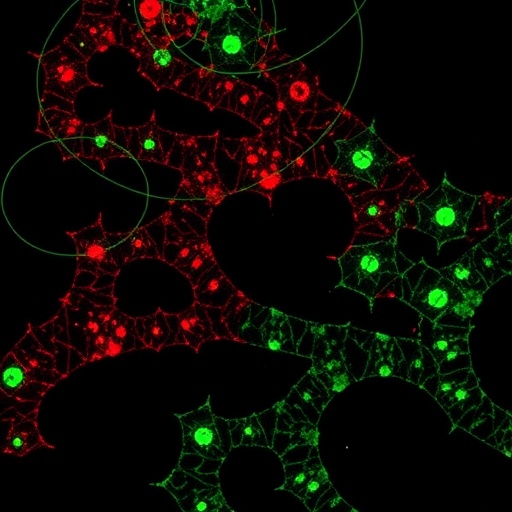
In the intricate world of cellular biology, the dynamic interplay between proteins and lipids within cellular membranes governs numerous vital processes fundamental to life. These interactions are notoriously transient and complex, challenging researchers to develop techniques capable of capturing and characterizing them with precision inside living cells. Breaking new ground in this arena, a team of scientists led by Becker et al. has unveiled a novel platform that elegantly bridges the gap between protein and lipid interactomics. This innovative approach, termed Photosensitizer Oxidative Crosslinking-based Proximity Labeling (POCA), harnesses the power of singlet oxygen chemistry to illuminate these elusive cellular conversations with unprecedented clarity.
Cellular membranes are not mere static barriers but dynamic landscapes where proteins and lipids continuously engage in fleeting yet critical interactions, influencing membrane structure, signaling, and trafficking. Conventional proximity labeling methods, however, encounter intrinsic limitations—they often require distinct and chemically divergent approaches to probe protein and lipid partners separately. Becker and colleagues have surmounted this challenge by conceptualizing and implementing a singular photocatalytic platform leveraging cell-penetrant photosensitizer molecules capable of generating singlet oxygen in situ. This reactive oxygen species activates proximal molecular partners, facilitating covalent tagging that marks both proteins and lipids concurrently.
The POCA technology capitalizes on the well-established HaloTag protein-labeling system, ensuring straightforward and versatile implementation within live cells. By conjugating photosensitizers to HaloTag substrates, researchers can direct singlet oxygen generation with high spatial and temporal control, effectively ‘highlighting’ neighboring biomolecules. In a landmark feat, this approach successfully captures interactomes involving cholesterol—a lipid known to play diverse and critical roles in membrane physiology. The cholesterol-directed POCA revealed not only previously characterized cholesterol-binding proteins but also identified novel interactors, shedding light on protein complexes whose association fluctuates with intracellular cholesterol dynamics.
The investigation into cholesterol-mediated interactions extends our comprehension of cellular lipid regulation, illuminating pathways sensitive to the bioavailability of cholesterol. Furthermore, the platform’s sensitivity to physiological contexts was underscored by its ability to detect proteins preferentially associated with cholesterol uptake through native lipoprotein pathways, a reflection of cellular adaptation to environmental lipid supply. These revelations underscore POCA’s capability to faithfully capture the nuances of lipid-protein interplay in live, physiologically relevant conditions, a feat that has eluded many prior methodologies.
Beyond cholesterol, protein-directed iterations of the POCA system were deployed to unravel the complex networks of intracellular membrane protein complexes. Utilizing this refined approach, the study dissected the interactome landscape of Aster-B, a cholesterol transport protein integral to maintaining sterol homeostasis. They discovered sterol-dependent alterations in Aster-B’s interaction partners, demonstrating that lipid composition intricately modulates protein complexes at the membrane interface. Remarkably, the study also uncovered singlet oxygen-driven domain-specific crosslinking events within Aster proteins, highlighting previously unappreciated mechanisms of protein interaction stabilization potentially governed by oxidative chemistry.
This domain-specific crosslinking phenomenon points to a broader biological implication, suggesting that oxidative post-translational modifications, either physiological or stress-induced, may influence protein assembly and function at membrane domains. The ability of POCA to map such nuanced variations could pave the way for new insights into redox biology and its impact on membrane protein behavior. The method’s finesse in capturing these spatially and temporally resolved interactions sets a new benchmark for proximity labeling technologies.
The development of POCA is a testament to interdisciplinary innovation, blending chemical biology, biophysics, and cell biology. Importantly, its design leverages cell-permeant photosensitizers that can be tethered selectively, enabling targeted singlet oxygen generation without widespread cellular damage—a critical consideration for maintaining cellular integrity during probing. This precision makes POCA highly adaptable for diverse research contexts, from fundamental biological discovery to potential therapeutic target identification.
Moreover, the ease of use and compatibility of the POCA framework with existing cellular labeling tools promises rapid adoption in various laboratories. Its dual capacity to tag both protein and lipid interactomes simultaneously sets it apart, addressing a long-standing need for holistic mapping of membrane-associated molecular networks. By uniting these traditionally separate spheres of interactomics, researchers gain a richer, more integrated picture of membrane biology.
This breakthrough not only advances our understanding of membrane-associated biology but also offers a versatile platform to explore pathological states where protein-lipid interactions play pivotal roles, such as in neurodegeneration, cardiovascular disease, and cancer. The ability to monitor changes in interactomes under different physiological conditions or pharmacological interventions opens new research vistas for precision medicine approaches targeting membrane-associated dysfunctions.
Furthermore, the study highlights the importance of tailoring proximity labeling chemistries to the unique chemical environments of different biomolecules. Singlet oxygen-mediated labeling confers distinct advantages for probing hydrophobic lipid domains and transient protein assemblies, a contrast to traditional radical-based or enzymatic proximity labeling techniques. As the field progresses, integrating photochemical strategies like POCA with other molecular tools can deepen our understanding of cellular architecture and dynamics.
The future implications of this work are vast. POCA’s fundamentally innovative methodology can be extended to diverse membrane types, including organellar membranes and cellular junctions, revealing context-dependent interactomes that shape cellular physiology. Additionally, the technology holds promise for high-resolution temporal studies, capturing rapid interaction dynamics previously inaccessible due to technical constraints.
In sum, Becker et al.’s POCA platform represents a significant leap forward in membrane interactomics, marrying chemical ingenuity with biological relevance. This singlet oxygen-based photocatalytic proximity labeling not only bridges a critical methodological divide but also unveils new biological insights into cholesterol biology, protein complex assembly, and oxidative crosslinking phenomena. As researchers worldwide embrace and build upon this tool, the cellular membrane—once viewed solely as a passive boundary—may now be appreciated as a vibrant, intricately orchestrated hub of molecular interplay.
The intersection of photochemistry and cell biology heralded by POCA thus sets a transformative precedent. By harnessing light-driven chemistry to illuminate cellular interactions in their native milieu, this approach reinvents proximity labeling for the next generation of discovery, promising to reshape our molecular understanding of the living cell’s most fundamental interface.
Subject of Research: Development of a singlet oxygen-based photocatalytic proximity labeling platform (POCA) to capture both protein and lipid interactomes within cellular membranes.
Article Title: Photosensitizer proximity labeling captures the lipid and protein interactomes.
Article References:
Becker, A.P., Biletch, E., Kennelly, J.P. et al. Photosensitizer proximity labeling captures the lipid and protein interactomes. Nat Chem Biol (2026). https://doi.org/10.1038/s41589-026-02140-1
Image Credits: AI Generated
DOI: https://doi.org/10.1038/s41589-026-02140-1
Tags: cell-penetrant photosensitizerscellular membrane interactionslipid signaling pathwayslipid-protein interactomeslive-cell interactome mappingmembrane protein dynamicsoxidative crosslinking proximity labelingphotocatalytic proximity labelingphotosensitizer labelingPOCA technologyprotein-lipid crosslinkingsinglet oxygen chemistry