Credit: Image courtesy: Tatsuo Kaneko and Mohammad Asif Ali from Japan Advanced Institute of Science and Technology.
Point:
- Novel chiral diacid monomers were synthesized.
- Chirally interactive BioNylons were prepared.
- BioNylon showed thermal/mechanical performances than conventional Nylons.
- BioNylons disintegrated and degraded with pepsin.
Summary:
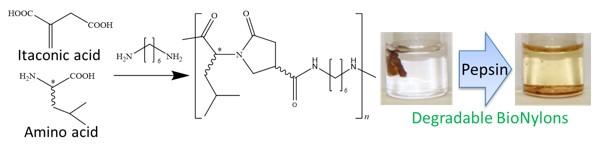
Marine plastic waste problems have been more serious year by year. One of the worst issues is that creatures in ocean are going extinct by mistakenly swallowing them.. Conventional biodegradable plastics are degradable in digestive enzymes, but their performances are too low to use in society. In this study, researchers from JAIST have used bio-derived resources such as itaconic acid and amino acid for the syntheses of high-performance BioNylons having the pepsin degradation function.
Ishikawa, Japan – Currently available conventional nylon such as Nylon 6, Nylon 66, and Nylon 11 are nondegradable. On the other hand, BioNylons derived from itaconic acid showed higher performances than conventional ones and degradability in soil, but degradability under the digestive enzymes was not confirmed.
To tackle these issues, a team of researchers from the Japan Advanced Institute of Science and Technologies (JAIST) are investigating syntheses of new BioNylons with their degradability under pepsin enzyme. Their latest study, published in Advanced Sustainable Systems-Wiley-VCH on April 2021, was led by Professor Tatsuo Kaneko and Dr. Mohammad Asif Ali.
In this study, BioNylons were synthesized based on chemically developed novel chiral dicarboxylic acids derived from renewable itaconic and amino acids (D- or L-leucine). Further, BioNylons were prepared via melt polycondensation of hexamethylenediamine with chirally interactive heterocyclic diacid monomers, as shown in Figure 1. The chiral interactions were derived from the diastereomeric mixture of the racemic pyrrolidone ring and the chiral amino acids of leucine. As a result, the polyamides showed a glass transition temperature, Tg, of approximately 117 °C and a melting temperature, Tm, of approximately 213 °C, which were higher than those of conventional BioNylon 11 (Tg of approximately 57 °C). The BioNylons also showed high Young’s moduli, E, and mechanical strengths, σ, ranging from 2.2-3.8 GPa and 86-108 MPa, respectively. Such materials can be used for fishing nets, ropes, parachutes, and packaging materials, as a substitute for conventional nylons. The BioNylons including peptide linkage showed enzymatic degradation using pepsin, which is a digestive enzyme found in mammal stomach. The fact that pepsin-degradation can connect with biodegradation in the stomach of marine mammals. Such an innovative molecular design for high-performance nylons by controlling chirality can lead to establish a sustainable carbon negative society and energy conservation by weight saving.
###
This research was carried out with the support of “Environment Research and Technology Development Fund (1-2005) of Environmental Restoration and Conservation Agency, ERCA. (Principal Investigator: Prof. Tatsuo Kaneko).
Media Contact
Tatsuo Kaneko
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




