Credit: Miquel Pons, University of Barcelona
Bacterial resistance does not come just through adaptation to antibiotics, sometimes the bacteria simply go to sleep. An international team of researchers is looking at compounds that attack bacteria's ability to go dormant and have found the first oxygen-sensitive toxin antitoxin system.
"Antibiotics can only kill bacteria when they are actively growing and dividing," said Thomas K. Wood, professor of chemical engineering and holder of the Biotechnology Endowed Chair, Penn State. "But, environmental stress factors often turn on a bacterial mechanism that creates a toxin that makes the cell dormant and therefore antibiotic resistant."
Bacteria that form biofilms are often difficult to kill. They can react to environmental signals and produce a toxin that makes the cells go dormant. Antibiotics cannot target dormant cells.
One type of bacterium that does this lives in the gastrointestinal track. Bile, secreted by the liver and stored in the gall bladder, when released into the GI track can kill bacteria. In the presence of bile, these bacteria produce a protein that is a self-toxin and the bacteria go dormant. When the bile is gone, the bacteria produce another protein that destroys the inhibitor protein and the bacteria come alive. These toxin antitoxin systems are inherent in bacteria and serve to protect them against a variety of external, environmental insults.
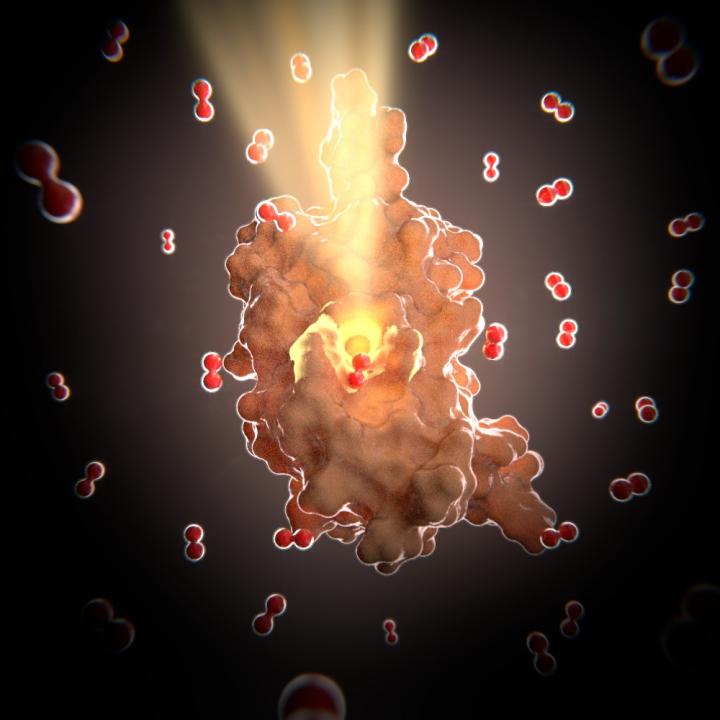
Wood and his colleagues characterized the first toxin antitoxin system in a biofilm. They report today (Dec. 8) in Nature Communications that this system also is the first known to be oxygen-dependent. The characterization was done at the molecular and atomic level by researchers at the Biomolecular NMR Laboratory at the University of Barcelona, Spain. They found that the E. coli antitoxin's structure had channels that are just large enough for oxygen to pass through. The toxin in this system is Hha and the antitoxin is TomB. However, unlike other toxin antitoxin pairs where the toxin makes the cell dormant and the antitoxin inactivates the toxin by binding, this system needs oxygen in the presence of the antitoxin to oxidize the toxin and wake up the bacteria.
"If we understand the toxin antitoxin systems at a molecular or atomic level, we can make better antimicrobials," said Wood. "I would argue that the toxin antitoxin systems are fundamental to the physiology of all bacteria. We hope this will give us insight into how they survive the antibiotics."
Free-swimming bacteria are usually easily targeted by antibodies or antibiotics, but bacteria that form biofilms are harder to kill. In tuberculosis, the bacteria have as many as 88 different toxin options to react to environmental stresses. According to Wood, this is one of the reasons that TB patients need to stay on antibiotics for months or years to clear the body of all the bacteria.
Biofilms are involved in 80 percent of human infections and are one of the strongest contributors to the pressing antibiotic resistance problem.
The researchers found that 10 percent oxygen is sufficient to wake up the bacteria, but in a biofilm, the problem becomes accessibility. The bacteria on the edges of the film can be easily exposed to oxygen, but those further inside the film might not come into contact with the oxygen. The channels that form in the E. coli biofilm allow the oxygen to penetrate into the biofilm, awaken the bacteria, break up the biofilm and disperse it.
The researchers suggest that this type of toxin, one that is oxygen-dependent, could become the target for antibacterial treatments to inhibit the formation of biofilms.
###
Also working on this project at Penn State were W.C. Soo, postdoctoral fellow, and Thammajun L. Wood, research associate in chemical engineering.
Other researchers included Oriol Marimon, Joáo M.C. Teixeira, Tiago N. Cordeiro, Irene Amata, Jesús Garcia, Ainara Morera and Miquel Pons, all at Biomolecular NMR Laboratory, Inorganic and Organic Chemistry Department, University of Barcelona, Spain; Maxim Mayzel, and Vladislave Yu. Orekhov, Swedish NMR Centre, Gothenburg University, Gothenburg, Sweden; and Marina Gay and Marta Vilaseca, Institute for Research in Biomedicine, The Barcelona Institute of Science and Technology, Barcelona, Spain.
The Army Research Laboratory, Spanish MINECO, EC FP7 BioNMR project supported this work.
Media Contact
A'ndrea Elyse Messer
[email protected]
814-865-9481
@penn_state
http://live.psu.edu
############
Story Source: Materials provided by Scienmag




