According to textbooks, proteins work by folding into stable 3D shapes that, like Lego blocks, precisely fit with other biomolecules.
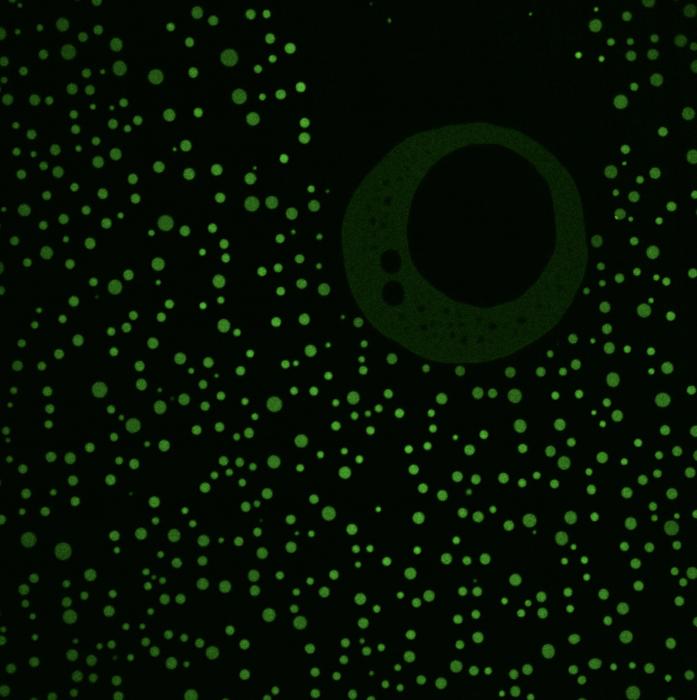
Credit: Amy Strom, Princeton University
According to textbooks, proteins work by folding into stable 3D shapes that, like Lego blocks, precisely fit with other biomolecules.
Yet this picture of proteins, the “workhorses of biology,” is incomplete. Around half of all proteins have stringy, unstructured bits hanging off them, dubbed intrinsically disordered regions, or IDRs. Because IDRs have more dynamic, “shape-shifting” geometries, biologists have generally thought that they cannot have as precise of a fit with other biomolecules as their folded counterparts, and as such, assumed these thread-like entities may contribute less significantly to overall protein function.
Now, a multi-institutional collaboration has uncovered how a key aspect of cell biology is controlled by IDRs. Their study, published Oct. 2 in the journal Cell, reveals that IDRs have specific and important interactions that play a central role in chromatin regulation and gene expression, essential processes across every living cell.
The researchers focused on disordered regions of the human cBAF complex, a multi-component group of proteins in the nucleus that works to open up the densely coiled-up DNA inside cells called chromatin, enabling genes along DNA to be expressed and turned into proteins. Mutations in the IDRs of one family of cBAF subunits, ARID1A and ARID1B, which are highly frequent in cancer and neurodevelopmental disorders, throw chromatin remodeling and gene expression out of whack, suggesting IDRs are anything but trivial extras.
In particular, the study revealed that the IDRs form little droplets called condensates that separate out from surrounding cellular fluid, just like drops of oil in water. The specific interactions that happen in these condensates allow proteins and other biomolecules to congregate in particular locations to carry out cellular activities. While scientists have shown that condensates perform a myriad of tasks, it was not known if these special liquid droplets had any role in chromatin remodeling, nor whether their specific amino acid sequences served specific functions.
Researchers from Princeton, the Dana-Farber Cancer Institute and Washington University in St. Louis teamed up to study the effects of different mutations in the ARID1A/B IDRs on the ability of the cBAF protein complex to form condensates and recruit partner proteins needed for gene expression. Some of the mutations examined in the study have been implicated in cancer or neurodevelopmental disorders. The results provide insights into how these mutations cause cellular processes to go awry, and could form the basis for novel therapeutic strategies.
“For the first time, we’ve shown that intrinsically disordered regions are fundamentally important for operation of a key chromatin remodeling complex, the cBAF complex” said Amy Strom, co-lead author of the study. “Our findings should be applicable to IDRs in general and could have significant implications for how cells do everything they do.”
Strom is co-lead author along with Ajinkya Patil, a former doctoral student at Harvard Medical School. Strom is a postdoctoral researcher in the lab of co-senior author Clifford Brangwynne, Princeton’s June K. Wu ’92 Professor in Engineering and director of the Omenn-Darling Bioengineering Institute; and Patil worked in the lab of co-senior author Cigall Kadoch, associate professor of pediatric oncology at the Dana-Farber Cancer Institute and Harvard Medical School, whose lab has a long-standing interest in chromatin remodeling in human health and disease.
“The degree to which even subtle disease-associated perturbations in IDR sequences altered the function of this major chromatin remodeler along the genome was surprising, and led us to explore the basis of the specific changes down to amino acid grammar,” said Patil.
Brangwynne, whose lab has studied disordered sequences and their role in forming condensates for years, said “Intrinsically disordered regions are everywhere in the vast catalog of human and other organisms’ proteins, and they’re playing central roles in physiology and disease in ways we’re just starting to understand.”
“Our discoveries shine new light not only on the mechanisms of cBAF chromatin remodeling complexes, which are among top targets in oncology, but on the inherent nature of sequence specificity in to-date poorly understood IDR protein sequences” said Kadoch. “These findings provide new foundations of important relevance toward the therapeutic targeting of condensates and their constituents.”
The work was supported in part by the Howard Hughes Medical Institute, the Air Force Office of Scientific Research, the St. Jude Children’s Research Hospital, the Mark Foundation for Cancer Research, and the National Science Foundation.
Journal
Cell
DOI
10.1016/j.cell.2023.08.032
Method of Research
Experimental study
Subject of Research
Cells
Article Title
A disordered region controls cBAF activity via condensation and partner recruitment
Article Publication Date
2-Oct-2023
COI Statement
C.K. is the scientific founder, Scientific Advisor to the Board of Directors, Scientific Advisory Board member, shareholder, and consultant for Foghorn Therapeutics. C.K. also serves on the Scientific Advisory Boards of Nereid Therapeutics (shareholder and consultant), Nested Therapeutics (shareholder and consultant), Accent Therapeutics (shareholder and consultant) and Fibrogen (consultant) and is a consultant for Cell Signaling Technologies and Google Ventures (shareholder and consultant). C.P.B. is a scientific founder, Scientific Advisory Board member, shareholder, and consultant for Nereid Therapeutics. R.V.P. is a member of the Scientific Advisory Board for Dewpoint Therapeutics (shareholder and consultant). The other authors declare no competing interests. The authors have submitted a patent application related to this work.




