To understand how the brain functions and how its dysfunction causes diseases, modalities to modulate neuronal activity with high precision are needed. Brain stimulation modalities with millimeter precision usually activate multiple functional regions and cause unintended responses. Therefore, a neuromodulation tool with ultrahigh precision is needed for mapping the brain sub-regions by modulating a small population of the neurons. Electrical stimulation tools are a gold standard for neuromodulation studies and disease treatments, but the current leakage over several millimeters limits the precise control of targeting. Optogenetics provides an unrivaled sub-cellular spatial resolution and specificity in targeted cell types, yet the viral transfection has limited their applications in the human brain. The emerging transcranial focused ultrasound (tFUS) as a non-invasive neuromodulation method offers millimeter-level precision in various models. A low ultrasonic frequency of ~ 1 MHz or less is preferred in tFUS for high transcranial efficiency, but limits its spatial resolution at 1 to 5 millimeters. Thus far, non-invasive brain stimulation by focused ultrasound with a spatial resolution of 0.1 mm has not been demonstrated. Non-invasive non-genetic neuromodulation with ultrahigh precision remains a critical unmet need.
Credit: by Yueming Li, Ying Jiang, Lu Lan, Xiaowei Ge, Ran Cheng, Yuewei Zhan, Guo Chen, Linli Shi, Runyu Wang, Nan Zheng, Chen Yang, Ji-Xin Cheng
To understand how the brain functions and how its dysfunction causes diseases, modalities to modulate neuronal activity with high precision are needed. Brain stimulation modalities with millimeter precision usually activate multiple functional regions and cause unintended responses. Therefore, a neuromodulation tool with ultrahigh precision is needed for mapping the brain sub-regions by modulating a small population of the neurons. Electrical stimulation tools are a gold standard for neuromodulation studies and disease treatments, but the current leakage over several millimeters limits the precise control of targeting. Optogenetics provides an unrivaled sub-cellular spatial resolution and specificity in targeted cell types, yet the viral transfection has limited their applications in the human brain. The emerging transcranial focused ultrasound (tFUS) as a non-invasive neuromodulation method offers millimeter-level precision in various models. A low ultrasonic frequency of ~ 1 MHz or less is preferred in tFUS for high transcranial efficiency, but limits its spatial resolution at 1 to 5 millimeters. Thus far, non-invasive brain stimulation by focused ultrasound with a spatial resolution of 0.1 mm has not been demonstrated. Non-invasive non-genetic neuromodulation with ultrahigh precision remains a critical unmet need.
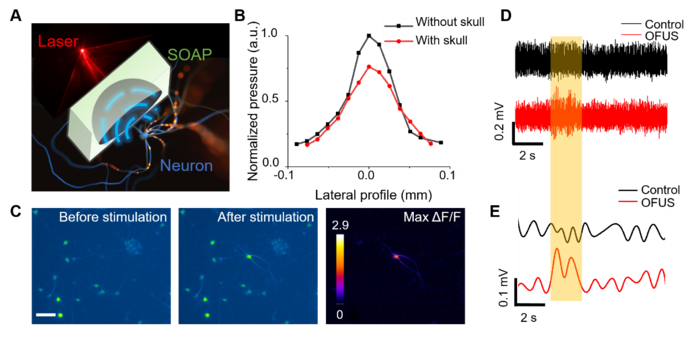
In a new paper published in Light Science & Application, a team of scientists and engineers, led by Professor Ji-Xin Cheng and Chen Yang from Boston University, USA, have developed optically-generated focused ultrasound (OFUS) for noninvasive brain stimulation with ultrahigh precision. OFUS is generated by a soft optoacoustic pad (SOAP) fabricated through embedding candle soot nanoparticles in a curved polydimethylsiloxane film. Through the optoacoustic effect, candle soot nanoparticles absorb the incident laser pulse and converts the energy to thermal expansion and compression, resulting in the generation of an ultrasound pulse. A transcranial focus with ultrahigh precision of 83 µm was achieved by SOAP, which is beyond the reach of the piezo-based low-frequency tFUS. Such a breakthrough benefited by the high acoustic frequency generated from the mixed layer of candle soot nanoparticles and PDMS, and was pushed towards the limit through the geometric design with a high numerical aperture. OFUS enables direct and transcranial single-cycle stimulation reliably and safely, verified by calcium imaging in cultured neurons in vitro. The unique single-cycle modulation of OFUS was found to evoke action potentials with much lower total ultrasound energy input than that of tFUS. The researchers also validated non-invasive transcranial neurostimulation with OFUS in mice by immunofluorescence imaging and electrophysiological recording. Therefore, OFUS offers ultrahigh precision non-invasively towards neurological research in sub-regions of a brain.
Notably, OFUS show the exciting potential for disease treatment, such as modulating complex brain function and histotripsy. To evoke complex functions of brain, OFUS device can be scaled up into a massive array for multisite neuromodulation. While the conventional PZT-based ultrasound arrays with massive cables connected to each element are heavy to wear for patients, the light-weight OFUS device also provides improved accessibility and wearability for long-term treatments. In addition, OFUS devices, with no metal components, further offer improved compatibility with real-time magnetic resonance imaging (MRI) guidance and functional MRI monitoring. These features of OFUS enable real-time fMRI evaluation of stimulation treatment and provide opportunities for close-loop treatments in clinical applications. Furthermore, OFUS offers an opportunity to improve spatiotemporal control in histotripsy. Conventional transducers for histotripsy are driven under thousands of volts, suffering from the risk of dielectric breakdown. OFUS can improve the spatial control with a higher frequency, deliver a better temporal precision with single cycle, and provide ultrasound with high intensity by simply improving the energy of input light without the risk of dielectric breakdown. The unique characteristics highlight a future direction of OFUS application in ultrasound surgery with improved spatiotemporal control, minimized damage, and heat accumulation to surrounding tissues.
Journal
Light Science & Applications
DOI
10.1038/s41377-022-01004-2




