Metabolic dysfunction-associated steatohepatitis (MASH) represents a complex and progressive liver disease, which often remains asymptomatic until advanced stages, posing considerable threats to global public health. Affecting approximately 30% of the world’s population, MASH not only increases the likelihood of cirrhosis but also raises the risk of hepatocellular carcinoma—a form of liver cancer that can be highly aggressive and lethal. The transition from a relatively benign condition of simple steatosis, characterized by lipid accumulation in the liver, to more severe manifestations such as inflammation, cell injury, fibrosis, and ultimately, malignancy underscores the urgent need for deeper insights into MASH pathogenesis. Understanding the underlying cellular mechanisms governing MASH is critical in identifying effective therapeutic interventions.
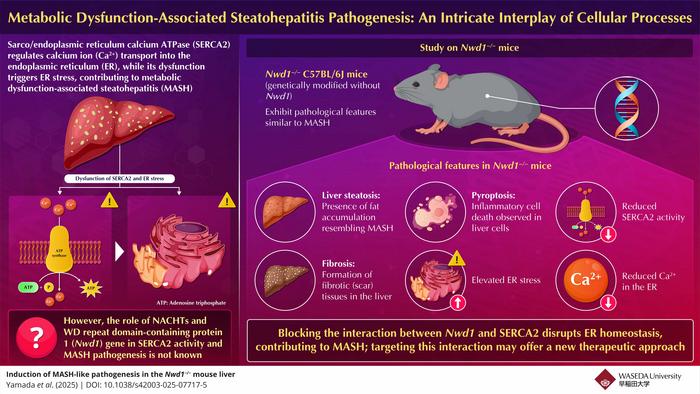
A significant aspect of MASH is the disruption of endoplasmic reticulum (ER) homeostasis, a crucial cellular compartment responsible for protein synthesis, folding, and lipid metabolism. The ER plays a pivotal role in calcium ion (Ca2+) storage and signaling, which is fundamental to its operational integrity. When the balance of folded and unfolded proteins is disturbed—often due to genetic factors, excess nutritional intake, or environmental stressors—the ER responds through a complex series of mechanisms known as ER stress. Chronic ER stress has been implicated in the pathophysiology of numerous metabolic disorders, including MASH. Recent investigations have begun to elucidate the significant role of sarco/ER calcium ATPase (SERCA2), a critical protein that mediates calcium transport within the ER, in maintaining this delicate balance. Dysfunction of SERCA2 has been linked to heightened ER stress, providing a possible nexus between calcium dysregulation and the advancement of MASH.
Delving into the genetic aspects of MASH, researchers have focused their attention on the NACHT and WD repeat domain-containing protein 1 (Nwd1) gene, known to be pivotal in various cellular functions, including signal transduction and ER dynamics. This gene is expressed in significant levels in both hepatic and central nervous tissues, yet its role in the context of liver pathogenesis associated with MASH has remained obscure until recently. One of the most intriguing revelations surrounding Nwd1 is its potential interaction with SERCA2, hinting at a collaborative relationship that might regulate ER function and overall liver homeostasis.
A recent publication in the journal Communications Biology has shed light on this interaction, as a team, led by Professor Shin-ichi Sakakibara from Waseda University in Japan, has undertaken a comprehensive study exploring the physiological implications of Nwd1 deletion in the context of MASH. The publication is significant not only for its exploration of Nwd1’s role but also for its broader implications in understanding the multifaceted nature of liver diseases, particularly in how genetic factors may influence the risk and progression of metabolic disorders.
Employing CRISPR-Cas9 genome editing technology, the research team created a knockout model devoid of Nwd1 (Nwd1−/− mice). These genetically modified mice were then subjected to extensive evaluation to ascertain the implications of Nwd1 deficiency on liver functionality and cellular processes. The findings were compelling; the absence of Nwd1 led to pronounced liver abnormalities characterized by severe lipid accumulation, fibrosis, and a marked increase in ER stress—phenomena that strikingly mirror the features observed in human MASH patients. Moreover, the study also found an alarming uptick in pyroptosis, a form of inflammatory cell death characterized by the activation of caspase-1, indicating a stark enhancement of hepatic inflammation and resultant tissue damage.
The data presented by Dr. Seiya Yamada, the first co-author of the study, provided significant insights into the regulatory role of Nwd1. The research disclosed that Nwd1 operates not in isolation but as a crucial regulator of ER stress mechanisms that are integral to maintaining calcium homeostasis within the liver. The deficiency of Nwd1 severely hampered SERCA2 activity, resulting in diminished Ca2+ storage capabilities of the ER, which in turn exacerbated the cellular stress response and facilitated lipid droplet accumulation—a hallmark of MASH.
The implications of these findings reach far beyond just theoretical significance. They suggest that targeting ER stress pathways may provide a viable strategy for developing new, much-needed therapies aimed at treating MASH. As Dr. Yamada pointed out, the mechanisms driving MASH are still not fully understood, and current therapeutic offerings are limited to a single approved drug. This gap in effective treatment options amplifies the urgency for research focused on identifying molecular targets that can be manipulated to ameliorate MASH progression.
In summary, the work led by Dr. Sakakibara and his colleagues presents a pivotal contribution to the understanding of MASH pathogenesis. By conceptualizing Nwd1 as a critical regulator within the ER calcium transport pathway, the study opens avenues for future research aimed at unraveling the complexities of liver diseases grounded in metabolic dysfunction. Importantly, as the prevalence of MASH continues to rise globally, advances in our understanding of its mechanistic underpinnings could lead to innovative therapeutic approaches that may ultimately reduce the burden of this disease.
In conclusion, the investigation into the role of Nwd1 in MASH represents a significant advance in our understanding of liver diseases and highlights the potential of gene-targeted therapies. The revelations from this study underscore the importance of continued exploration of molecular pathways involved in metabolic disorders. As researchers continue to piece together the intricate puzzle of MASH, the hope is that such insights will lead to effective treatment strategies that can transform the landscape of liver disease management and improve patient outcomes across diverse populations.
Subject of Research: Animals
Article Title: Induction of MASH-like pathogenesis in the Nwd1−/− mouse liver
News Publication Date: 11-Mar-2025
Web References: https://doi.org/10.1038/s42003-025-07717-5
References: Communications Biology
Image Credits: Professor Shin-ichi Sakakibara from Waseda University, Japan
Keywords: Metabolic dysfunction, liver disease, steatohepatitis, ER stress, Nwd1, SERCA2, therapeutic targets.
Tags: cellular mechanisms in liver diseasechronic ER stress implicationsendoplasmic reticulum homeostasis disruptiongenetic factors in metabolic disordershepatocellular carcinoma riskslipid metabolism and liver healthliver disease research advancementsMASH-like pathology in micemetabolic dysfunction-associated steatohepatitisNwd1 gene knockoutpublic health impact of liver diseasestherapeutic interventions for MASH