Unlocking Cellular Health: How Spns1 Facilitates the Recycling of Lipid Molecules
In an era where cellular health is becoming increasingly vital, researchers from the Yong Loo Lin School of Medicine at the National University of Singapore (NUS Medicine) have unveiled groundbreaking insights into the intricate mechanisms governing lipid recycling within cells. Their study primarily revolves around a protein known as Spinster homolog 1 (Spns1), which plays a pivotal role in transporting fat molecules out of lysosomes, the cell’s recycling hub.
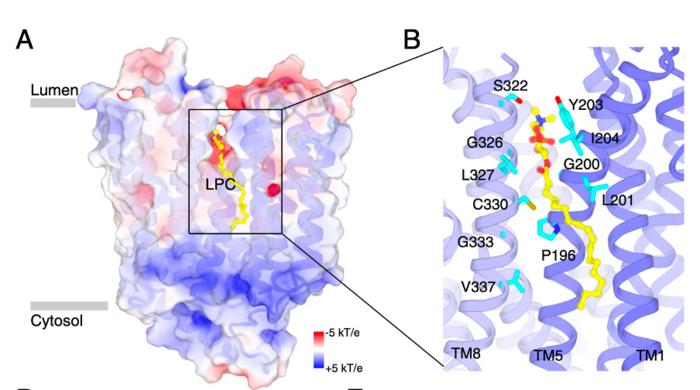
Lysosomes are often referred to as the cell’s digestive system, where they meticulously break down various biomolecules, including fats, to recycle them for essential cellular functions. The research demonstrates that Spns1 acts as a molecular gatekeeper, overseeing the efficient transfer of lysophospholipids—specifically lysophosphatidylcholine (LPC)—from the lysosome to the cytosol. This process ensures that valuable lipid components are not wasted, preventing the detrimental accumulation of fat molecules within cellular compartments.
The study utilized advanced imaging techniques like cryoelectron microscopy to observe the interaction dynamics between Spns1 and LPC. The detailed visualization provided researchers with a granular understanding of how Spns1 operates, including its ability to respond to environmental cues that dictate its activity. This newfound knowledge about Spns1’s function could potentially pave the way for novel therapeutic approaches targeting lysosomal storage disorders.
Each day, cells engage in a relentless battle to maintain their health by meticulously managing their lipid content. The breakdown of fats within lysosomes is not merely a cleanup operation but rather a cornerstone for numerous cellular functions. After fats are metabolized, their constituent parts—such as phospholipids and sphingolipids—are essential in rebuilding and maintaining cellular membranes. Furthermore, fats serve as critical energy sources and contribute to intercellular signaling, a process vital for ensuring cell survival and proper function.
A critical association exists between the dysregulation of lipid transport and the manifestation of lysosomal storage diseases (LSDs). These rare genetic disorders arise when lysosomal degradation fails, leading to the accumulation of waste products, often fat-based. Notably, diseases such as Gaucher, Tay-Sachs, and Niemann-Pick, as well as neurodegenerative diseases like Parkinson’s and Alzheimer’s, highlight the crucial link between dysfunctional lipid metabolism and human health.
The investigative team, led by Associate Professor Nguyen Nam Long, has generated significant momentum in understanding Spns1’s role as a facilitator of lipid export from lysosomes. The study’s findings indicate that Spns1 can open and close in response to specific signals, effectively mediating the egress of fats from the lysosome. The implications of this regulatory mechanism are profound, suggesting that understanding Spns1’s signaling pathways could unlock new strategies for ameliorating lysosomal storage disorders.
In addition to their core findings, the researchers delved into the mechanisms by which mutations in the Spns1 gene could adversely affect lipid transport, leading to clinical manifestations of disease. Identifying and understanding these mutations could serve as a critical avenue for developing targeted therapies aimed at correcting or compensating for the dysfunction observed in LSDs.
Furthermore, the study emphasizes the significance of intercellular communication in maintaining cellular health. Lipid molecules, such as sphingosine-1-phosphate (S1P), facilitate signaling pathways that oversee essential processes ranging from cell growth to apoptosis. The multifaceted roles of lipids underscore the necessity for precise control during cellular lipid recycling processes, highlighting how proteins like Spns1 integrate into a broader biochemical network.
Beyond elucidating Spns1’s function, the research team’s collaboration with experts from the University of Texas Southwestern Medical Center has pioneered new methodologies for visualizing lipid transport mechanisms at the molecular level. The utilization of cryo-EM offered unparalleled insights into protein-lipid interactions, enabling the unraveling of the molecular intricacies governing cellular lipid homeostasis.
As science moves toward a more comprehensive understanding of pathophysiology, the implications of this research extend into future therapeutic landscape. The authors noted that developing small molecules to target the activity of Spns1 could yield potential treatments for LSDs, marking a significant step forward in the field of molecular medicine.
This research adds a compelling chapter to the ongoing discourse surrounding cellular metabolism and disease prevention. By deepening our understanding of how proteins like Spns1 function, we move closer to unveiling new treatment options for patients suffering from lysosomal storage diseases and other lipid metabolism disorders. The commitment to uncovering these cellular secrets speaks to a hopeful future where innovative therapies can arise from fundamental biological discoveries.
As the research community hails this pivotal study, it remains eager to see how these insights will shape our understanding of cellular health and disease management in years to come. The pursuit of knowledge surrounding lipid transport mechanisms not only enriches the scientific narrative but also holds the promise of tangible improvements in human health outcomes.
With the potential for further exploration into Spns1’s transport cycles and activities, researchers continue to aim for a holistic understanding of lysosomal function. The delicate dance of lipids within and outside the cell continues to symbolize the complexity of life at the cellular level.
Subject of Research: Spinster homolog 1 (Spns1) and its role in lipid transport from lysosomes
Article Title: Molecular basis of Spns1-mediated lysophospholipid transport from the lysosome
News Publication Date: 31-Dec-2024
Web References: Proceedings of the National Academy of Sciences
References: 10.1073/pnas.2409596121
Image Credits: NUS Medicine
Keywords: Cellular biology, Lipid metabolism, Lysosomal storage diseases, Protein function, Molecular biology, Lipid signaling.
Tags: advanced imaging techniques in biologycellular health and diseasecryoelectron microscopy applicationsenvironmental cues in cellular activityfat accumulation in cellsimplications for disease risk managementlipid recycling mechanismslysophosphatidylcholine transportlysosome cellular processesmolecular gatekeeper roleNUS Medicine researchSpns1 protein function