Credit: Insilico Medicine
- A study published in Nature Biotechnology presents the entire journey of INS018_055, from AI algorithms to Phase II clinical trials for the first time.
- Raw data from 13 preclinical experiments and 3 clinical trials referenced in this study can be accessed by visiting Insilico’s data room.
- Insilico developed a PaperGPT system based on ChatGPT-4 Turbo and internal LLM that provides answers related to the paper via chat functionality.
There are thousands of diseases worldwide with no cure or available treatments. Traditional drug discovery and development takes decades and billions of dollars and more than 90% of these drugs fail in clinical trials. The emergence of artificial intelligence (AI) holds promise for streamlining and improving the entire process. However, ushering in a new era of AI-driven drug discovery requires costly and lengthy validation in preclinical cell, tissue, and animal models and human clinical trials.
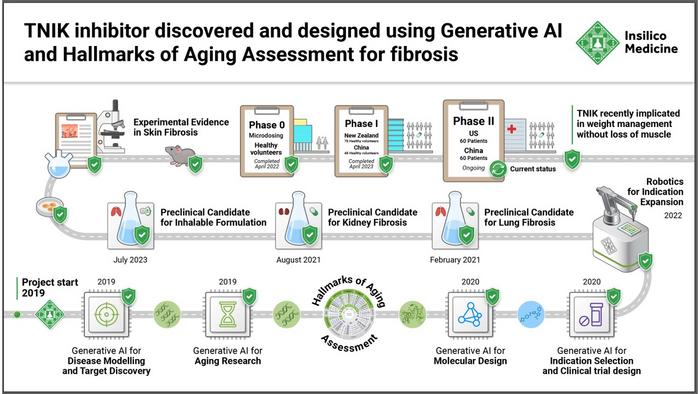
Now, that preclinical and part of that clinical validation was published in a new study in Nature Biotechnology. In this paper, Insilico Medicine and collaborators present the journey of its lead therapeutic program with an AI-discovered target and novel molecule generated from AI algorithms to Phase II clinical trials. For the first time, the paper discloses the raw experimental data and the preclinical and clinical evaluation of the potentially first-in-class TNIK inhibitor discovered and designed through generative AI. The study underscores the benefits of AI-led drug discovery methodology to provide efficiency and speed to drug discovery and highlights the promising potential of generative AI technologies for transforming the industry.
“When our first paper in the generative AI for generation of novel molecules was published in 2016, followed by many follow-up papers, the drug discovery community was very skeptical. Even after several validation experiments and launch of our AI software platform that is now used by many biopharma companies, many questions remained. Based on the research data, especially those from the live clinical program. To date, I have not seen anything close from any other company in our field,” said Alex Zhavoronkov, PhD, founder and CEO of Insilico Medicine. “From my perspective, the progress of INS018_055 has significant implications for the drug discovery field. It not only serves as a proof-of-concept for Pharma.AI, our end-to-end AI-driven drug discovery platform, but sets a precedent for the potential of generative AI to accelerate drug discovery. Using the publication as a guide, one can extrapolate how generative AI drug discovery tools may streamline early discovery efforts. We anticipate that the expanded application of this platform will address challenges facing industry R&D, including cost and efficiency, and accelerate the delivery of innovative therapies to patients.”
Insilico initiated the research by focusing on fibrosis, a biological process closely associated with aging. The group first trained PandaOmics, the target identification engine of Insilico’s proprietary AI platform Pharma.AI, on the collection of omics and clinical datasets related to tissue fibrosis. Next, PandaOmics proposed a potential target list using deep feature synthesis, causality inference, and de novo pathway reconstruction. After that, the natural language processing (NLP) models of PandaOmics analyzed millions of text files, including patents, publications, grants, and clinical trial databases to further assess the novelty and disease association. TNIK was identified as the most promising anti-fibrosis target. Notably, TNIK had been indirectly linked to multiple fibrosis-driven pathways in previous research but was never pursued as a potential target for IPF. In a separate paper, Insilico scientists demonstrated that TNIK may be implicated in multiple hallmarks of aging.
After selecting TNIK as a primary target, Insilico scientists utilize Chemistry42, the Company’s generative chemistry engine, to generate novel molecular structures with the desired properties using the structure-based drug design (SBDD) workflow. Chemistry42 combines over 40 generative chemistry algorithms and over 500 pre-trained reward models for de novo compound generation, and can optimize both generation and virtual screening based on expert human feedback. Following multiple iterative screens, one promising hit candidate demonstrated activity with nanomolar IC50 values. The group further optimized the compound to increase solubility, promote a good ADME safety profile, and mitigate unwanted toxicity while retaining its remarkable affinity for TNIK, which ultimately produced the lead molecule INS018_055, with less than 80 molecules synthesized and tested.
In subsequent preclinical studies, INS018_055 demonstrated significant efficacy in vitro and in vivo studies for IPF and showed promising results in pharmacokinetic and safety studies across multiple cell lines and multiple species. Furthermore, INS018_055 showed pan-fibrotic inhibitory function, attenuating skin and kidney fibrosis in two additional animal models. Based on these studies, INS018_055 achieved preclinical candidate nomination in February 2021, in less than 18 months following PandaOmics’ proposal of TNIK as a potentially novel target for IPF in 2019.
INS018_055 has exhibited excellent performance in clinical trials to date. In November 2021, 9 months after PCC nomination, the first healthy volunteers were dosed in a first-in-human (FIH) microdose trial of INS018_055 in Australia. This microdose trial exceeded expectations, delivering a favorable pharmacokinetic and safety profile that successfully demonstrated this clinical proof-of-concept and set the stage for the next step of clinical testing. In Phase I trials carried out in New Zealand and China, INS018_055 was tested in 78 and 48 healthy subjects, divided into cohorts focusing on a single ascending dose (SAD) study and multiple ascending dose (MAD) study. The international multi-site Phase I studies yielded consistent results, demonstrating favorable safety, tolerability, and pharmacokinetics (PK) profiles of INS018_055, and supporting the initiation of the Phase II studies.
“Combining AI methods with human intelligence, we have successfully nominated INS018_055, a potentially first-in-class antifibrotic inhibitor, with significant reductions in time and costs”, said Feng Ren, PhD, co-CEO and Chief Scientific Officer of Insilico Medicine. “Encouraged by positive preclinical and available clinical data, we look forward to favorable performance of INS018_055 in Phase 2 clinical trials, which would provide innovative options for patients while bringing more solid evidence for the AI-driven drug discovery industry.”
At the time of this publication, two Phase 2a clinical trials of INS018_055 for the treatment of IPF are being conducted in parallel in the United States and China. The studies are randomized, double-blind, placebo-controlled trials designed to evaluate the safety, tolerability and pharmacokinetics of the lead drug. In addition, the trials will assess the preliminary efficacy of INS018_055 on lung function in IPF patients. As this drug continues to advance, it drives hope for the roughly five million people worldwide suffering from this deadly disease.
Insilico’s drug discovery efforts are driven by its validated and commercially viable AI drug discovery platform, Pharma.AI, which works across biology, chemistry, and clinical medicine, providing the biotechnology and the pharmaceutical industry with advanced generative AI tools to accelerate their internal research and development. Powered by Pharma.AI, Insilico is delivering breakthroughs for healthcare in multiple disease areas, including fibrosis, cancer, immunology and aging-related disease. Since 2021, Insilico has nominated 18 preclinical candidates in its comprehensive portfolio of over 30 assets and has advanced six pipelines to the clinical stage.
Industry Commentary and Additional Information
“There has been much speculation that AI and deep learning methods will have a substantial role in shaping the future course of drug development. This paper presents a very convincing proof of concept.” says Charles Cantor, PhD, Scientific Advisory Board (SAB) at Insilico Medicine, “Driven by AI at nearly every stage from target identification to drug candidate selection, to phase 1 studies, a novel molecule is now ready for phase 2 clinical trials. If this process proves to be general, drug development without AI may well become inconceivable.”
“Healthcare is undergoing an important transformation of digitalization.” says Dr.Kai-Fu Lee Chairman of Sinovation Ventures, CEO of 01.AI. “I believe the use of AI and data science will revolutionize the field of medicine. Insilico Medicine’s TNIK program is a great example, presenting a breakthrough paradigm for discovering medicines from scratch under generative AI in chemistry and biology. The milestones achieved by Insilico, backed by compelling experimental data, will encourage the entire ecosystem that we are marching down the right track to advance life science with state-of-the-art information technology.”
“Although lots of companies are working on AI to improve different steps in drug discovery, Insilico is trying to apply their AI in all early drug discovery and design stages, which is so exciting to me,” says Michael Levitt, PhD, Nobel Laureate in Chemistry, 2013. “Insilico is literally AI from A to Z. They not only identified a novel target, but also accelerated the whole early drug discovery process and they’ve quite successfully validated their AI methods in the TNIK program. Drug discovery is a very wide-ranging project with a lot of uncertainty. AI can cope well with specific techniques for huge amounts of data, and by combining them with clever filtering, we can gain certainty and options from uncertainty.”
“Nowadays, it seems that we read about the virtues of AI, ML, generative design almost daily. There is a feeling that, perhaps, it is overhyped,” says Stevan Djuric, PhD, adjunct professor in medicinal chemistry at the University of Kansas and former head of the global medicinal chemistry leadership team at AbbVie. “However, in this paper, the Insilico Medicine team convincingly demonstrates the power of their proprietary platform which features target identification and validation, medicinal chemistry design and clinical trial components using the aforementioned tools. For experienced medicinal chemists, improving potency of compounds is often not the major challenge, but rather fine tuning of PK (CLu etc) and safety (off-target effects). The Insilico engine, in the case presented, successfully tackled all components of these difficult problems in a particularly timely manner. We will eagerly await news on the further progress of this agent and further clinical candidates discovered using generative AI paradigms.”
“Many people say they are doing AI for drug discovery,” says Alán Aspuru-Guzik, PhD, professor of Chemistry and Computer Science at the University of Toronto and director of the Acceleration Consortium. “A handful are delivering. Insilico’s team has shown both, the identification of a target followed by the development of a therapeutic agent all driven by AI. This, to my knowledge, is the first AI-generated drug in stage II clinical trials. A true milestone for the community and for Insilico. The lessons learned here can be further expanded to accelerate the discovery and development process.”
“When we started the preclinical profiling of INS018_055 for fibrotic diseases, everybody was seeing its target TNIK as a candidate for oncology indications,” says Klaus Witte, MD, German Medical Board certified Pharmacologist & Toxicologist, Preclinical Consultant to Insilico Medicine. “Although I was skeptical in the beginning, all data that we generated clearly supported Insilico’s prediction of anti-fibrotic efficacy. Looking at the broad set of preclinical and clinical data that is meanwhile available, I’m confident that INS018_055 could become a very valuable treatment option in pulmonary fibrosis and other fibrosis indications. Having helped to bring the compound to its present stage is something I’m really proud of.”
“As a clinician, I see firsthand the need for novel, effective treatments. This landmark work highlights the central role that AI can play in accelerating the path from discovery to treatment, reshaping our strategy against diseases that currently have limited therapeutic options,” says Prof. Christoph Kuppe, PhD, physician scientist at the RWTH Aachen.
“I was struck by the incredible progress Alex and Insilico have made in a single decade,” says Bud Mishra, PhD, professor of computer science at New York University. “The paper focuses on idiopathic pulmonary fibrosis, a disease with complex genetics involving multiple genetic mutations. They broke this complex problem down into two parts: selecting a target (namely, TNIK), and then guiding the drug discovery process by designing small molecules that would bind to that target and make it ineffective. The first part uses heuristics that are based on the scientific experiences accumulated in the past (the target must be novel, easy to understand in terms of interactions with known pathways, and follow the approaches used by others in guiding drug discovery and clinical trials in the past) and hence ideal for NLP using LLMs. The second part uses randomized heuristics to search and optimize over complex combinatorial spaces using DNNs, capable of dealing with naturally occurring ‘easy instances of hard problems.’ Speculatively, the first part will become more difficult over time (hallucination vs. true novelty) and the second part, simpler, as Moore’s law will continue to improve the computational power exponentially.”
Reference
Ren, F., et al. A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models. Nat Biotechnol (2024). https://doi.org/10.1038/s41587-024-02143-0
About Insilico Medicine
Insilico Medicine, a global clinical-stage biotechnology company powered by generative AI, connects biology, chemistry, and clinical trial analysis using next-generation AI systems. The company has developed AI platforms that utilize deep generative models, reinforcement learning, transformers, and other modern machine learning techniques for novel target discovery and generating novel molecular structures with desired properties. Insilico Medicine is developing breakthrough solutions to discover and develop innovative drugs for cancer, fibrosis, immunity, central nervous system diseases, infectious diseases, autoimmune diseases, and aging-related diseases. www.insilico.com
Journal
Nature Biotechnology
DOI
10.1038/s41587-024-02143-0
Article Title
A small-molecule TNIK inhibitor targets fibrosis in preclinical and clinical models
Article Publication Date
8-Mar-2024




