Antiviral therapies are notoriously difficult to develop, as viruses can quickly mutate to become resistant to drugs. But what if a new generation of antivirals ignores the fast-mutating proteins on the surface of viruses and instead disrupts their protective layers?
Credit: David Song/NYU
Antiviral therapies are notoriously difficult to develop, as viruses can quickly mutate to become resistant to drugs. But what if a new generation of antivirals ignores the fast-mutating proteins on the surface of viruses and instead disrupts their protective layers?
“We found an Achilles heel of many viruses: their bubble-like membranes. Exploiting this vulnerability and disrupting the membrane is a promising mechanism of action for developing new antivirals,” said Kent Kirshenbaum, professor of chemistry at NYU and the study’s senior author.
In a new study published Aug. 2 in the journal ACS Infectious Diseases, the researchers show how a group of novel molecules inspired by our own immune system inactivates several viruses, including Zika and chikungunya. Their approach may not only lead to drugs that can be used against many viruses, but could also help overcome antiviral resistance.
The urgent need for new antivirals
Viruses have different proteins on their surfaces that are often the targets of therapeutics like monoclonal antibodies and vaccines. But targeting these proteins has limitations, as viruses can quickly evolve, changing the properties of the proteins and making treatments less effective. These limitations were on display when new SARS-CoV-2 variants emerged that evaded both the drugs and the vaccines developed against the original virus.
“There is an urgent need for antiviral agents that act in new ways to inactivate viruses,” said Kirshenbaum. “Ideally, new antivirals won’t be specific to one virus or protein, so they will be ready to treat new viruses that emerge without delay and will be able to overcome the development of resistance.”
“We need to develop this next generation of drugs now and have them on the shelves in order to be ready for the next pandemic threat—and there will be another one, for sure,” added Kirshenbaum.
Drawing inspiration from our immune systems
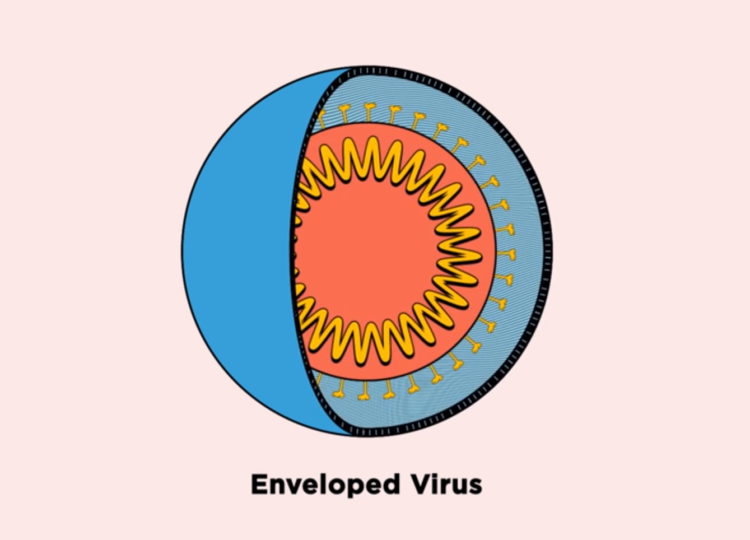
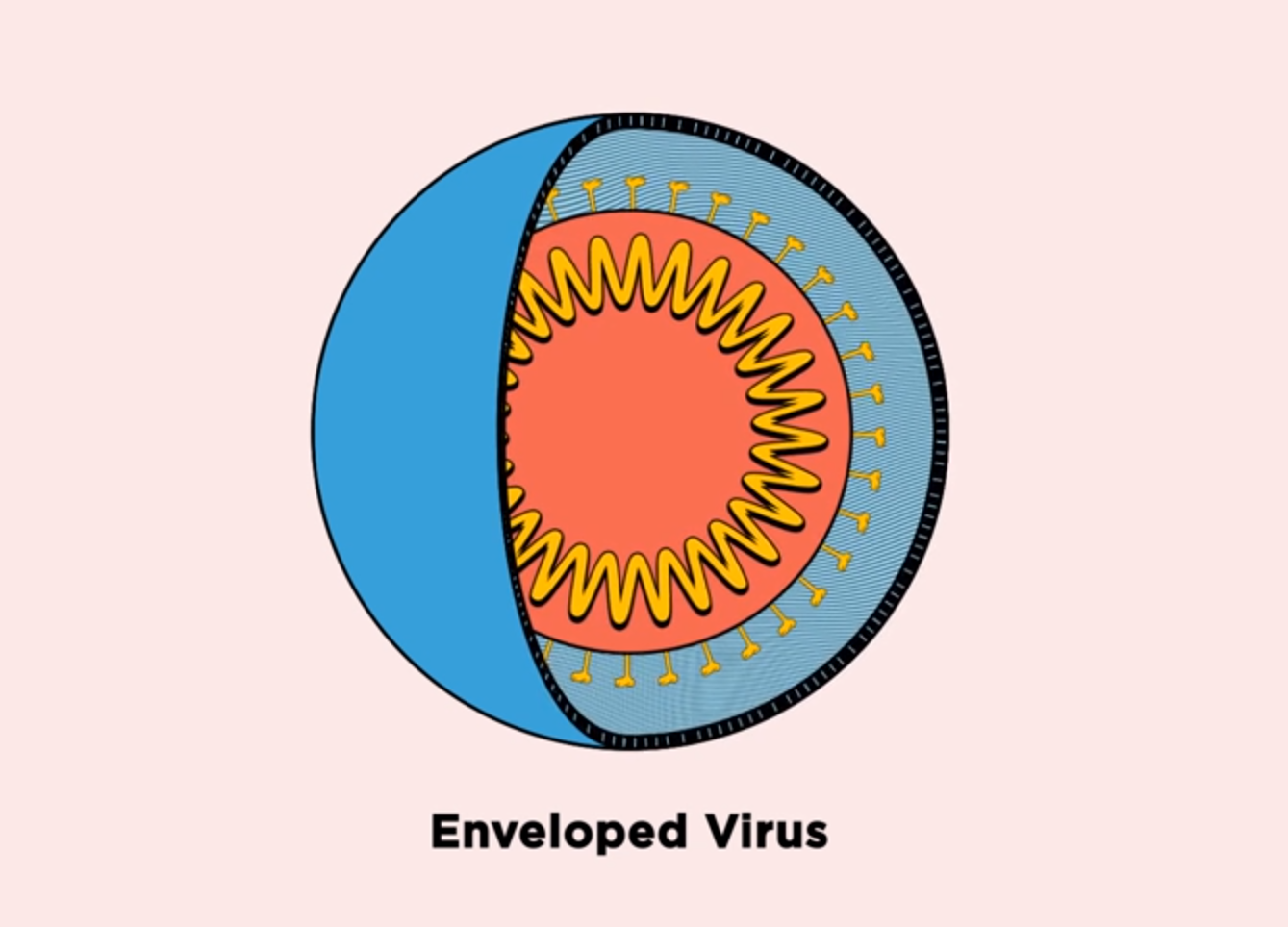
Our innate immune system combats pathogens by producing antimicrobial peptides, the body’s first line of defense against bacteria, fungi, and viruses. Most viruses that cause disease are encapsulated in membranes made of lipids, and antimicrobial peptides work by disrupting or even bursting these membranes.
While antimicrobial peptides can be synthesized in the lab, they are rarely used to treat infectious diseases in humans because they break down easily and can be toxic to healthy cells. Instead, scientists have developed synthetic materials called peptoids, which have similar chemical backbones to peptides but are better able to break through virus membranes and are less likely to degrade.
“We began to think about how to mimic natural peptides and create molecules with many of the same structural and functional features as peptides, but are composed of something that our bodies won’t be able to rapidly degrade,” said Kirshenbaum.
The researchers investigated seven peptoids, many originally discovered in the lab of Annelise Barron at Stanford, a co-author of the study. The NYU team studied the antiviral effects of the peptoids against four viruses: three enveloped in membranes (Zika, Rift Valley fever, and chikungunya) and one without (coxsackievirus B3).
“We were particularly interested in studying these viruses as they have no available treatment options,” said Patrick Tate, a chemistry PhD student at NYU and the study’s first author.
How peptoids disrupt viral membranes and avoid other cells
The membranes surrounding viruses are made of different molecules than the virus itself, as lipids are acquired from the host to form membranes. One such lipid, phosphatidylserine, is present in the membrane on the outside of viruses, but is sequestered towards the interior of human cells under normal conditions.
“Because phosphatidylserine is found on the exterior of viruses, it can be a specific target for peptoids to recognize viruses, but not recognize—and therefore spare—our own cells,” said Tate. “Moreover, because viruses acquire lipids from the host rather than encoding from their own genomes, they have better potential to avoid antiviral resistance.”
The researchers tested seven peptoids against the four viruses. They found that the peptoids inactivated all three enveloped viruses—Zika, Rift Valley fever, and chikungunya—by disrupting the virus membrane, but did not disrupt coxsackievirus B3, the only virus without a membrane.
Moreover, chikungunya virus containing higher levels of phosphatidylserine in its membrane was more susceptible to the peptoids. In contrast, a membrane formed exclusively with a different lipid named phosphatidylcholine was not disrupted by the peptoids, suggesting that phosphatidylserine is crucial in order for peptoids to reduce viral activity.
“We’re now starting to understand how peptoids actually exert their antiviral effect—specifically, through the recognition of phosphatidylserine,” said Tate.
The researchers are continuing pre-clinical studies to evaluate the potential of these molecules in fighting viruses and to understand if they can overcome the development of resistance. Their peptoid-focused approach may hold promise for treating a wide range of viruses with membranes that can be difficult to treat, including Ebola, SARS-CoV-2, and herpes.
In addition to Kirshenbaum, Tate, and Barron, study authors include Vincent Mastrodomenico, Christina Cunha, and Bryan C. Mounce of Loyola University Chicago Medical Center; Joshua McClure of Maxwell Biosciences; and Gill Diamond of the University of Louisville School of Dentistry.
The research was supported in part by the National Science Foundation (CHE-2002890 and NSF GRFP) and the National Institutes of Health (R35GM138199 and 1DP1 OD029517-01). Kirshenbaum is the Chief Scientific Officer for Maxwell Biosciences, a biotech company that has licensed patents originating from his lab at NYU. The company is seeking to commercialize these compounds and bring them to the clinic to advance human health.
Journal
ACS Infectious Diseases
DOI
10.1021/acsinfecdis.3c00063
Subject of Research
Cells
Article Publication Date
2-Aug-2023