Credit: by Jun Zhu, Hercules Rezende Freitas, Izumi Maezawa, Lee-way Jin, and Vivek J. Srinivasan
Central nervous system (CNS) diseases such as Alzheimer’s disease (AD) manifest early at the microscopic (i.e. cellular) level, deep in the brain. Yet, optical microscopes that can see cells in the living brain are superficial or invasive. Whole brain imaging techniques such as magnetic resonance imaging are deep and non-invasive, but lack cellular resolution.
In a new paper published in Light Science & Application, a team of scientists, led by Professor Vivek J. Srinivasan from the Departments of Ophthalmology and Radiology and Tech4Health Institute, NYU Langone Health, USA, and co-workers have developed a label-free optical microscopy approach that has a unique ability to image deep, with high resolution and minimal invasiveness. Specifically, they demonstrated an in vivo high numerical aperture optical coherence microscopy (OCM) approach that utilizes the 1700 nm water absorption window, where attenuation of light by scattering and absorption is minimized.
The 1700 nm water absorption window, also known as the third near-infrared (NIR) window, boasts a local water absorption minimum and relatively low scattering. In OCM, a broader spectrum provides a finer axial resolution, and with it, a stronger ability to reject multiply scattered light that causes image blur. Yet the entire 1700 nm window, which spans from 1560 to 1820 nm, is often not used:
“The transition from standard wavelengths to 1700 nm OCM, while optimally using the entire water absorption window (not just a portion of the window), has been very difficult to date due to the numerous optical engineering challenges.” the scientists mentioned.
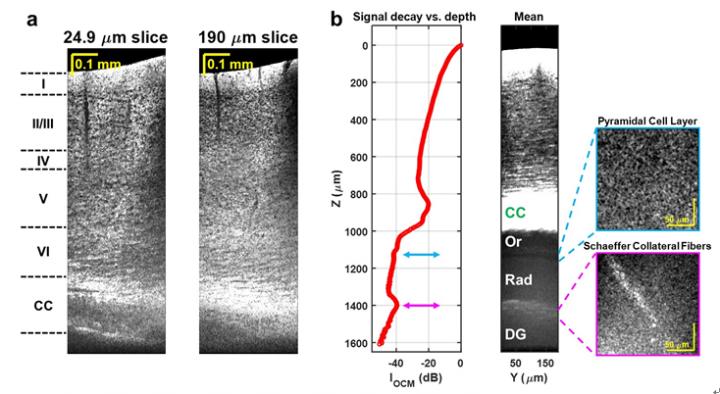
These challenges include noisy detectors and light sources, severe chromatic dispersion, and lack of standardized optical components. The scientists addressed these issues through the choice of a low noise supercontinuum light source, a custom numerical dispersion compensation method, and optical system design. With these technical advances, neuronal cell and myelin architecture across the entire depth of the mouse neocortex, and some sub-cortical regions, can be imaged through a thinned-skull preparation that preserves intracranial space.
“The results represent unprecedented depths for cellular-scale brain imaging through a minimally invasive preparation. We next investigated the 5xFAD mouse model of Alzheimer’s disease (AD), which is expected to show a gradation of pathology with cortical depth. The imaging results confirmed the appearance of severe pathology in deep but not superficial cortex, which would be missed by more superficial imaging techniques.”
Another important feature of the method is that the image contrast arises from intrinsic properties of the brain itself. OCM does not require transgenic mice or administration of compounds. Neuronal cell body loss, demyelination of axons, plaques, and local tissue changes can all be imaged.
“Now disease can be visualized deep in the mouse brain with a simple surgical preparation, without exogenous labeling. The 1700 nm optical window can also quantify tissue water and lipid content in vivo, which may provide further insights into disease progression.” the scientists forecast.
###
Media Contact
Jun Zhu
[email protected]
Related Journal Article
http://dx.




