Understanding the mechanisms that impair lymphatics growth and fat digestion in the small intestine
Credit: IBS
You have just enjoyed a delicious summer BBQ. After approximately eight hours, food molecules reach your small intestine, where specialized lymph capillaries, called lacteals, absorb fat nutrients. Lacteals are different from other lymphatics, as they continue to regenerate during adulthood, with a slow, but steady pace. Their unique renewal capacity is still poorly understood.
A team of scientists led by KOH Gou Young at the Center for Vascular Research, within the Institute for Basic Science (IBS, South Korea) have identified new subsets of gut connective cells, which are crucial for lymphatic growth. Their new findings have been reported in the journal Nature Communications.
The walls of the small intestine are covered with fingerlike projections, called villi. Lining these villi, heterogeneous populations of epithelial, immune, vascular, connective and even neural cells co-exist and help the digestive process. Lacteals and blood capillaries run inside the villi and take in different food molecules. The gut environment needs to cope with water secretion and reabsorption (osmotic stress), as well as the repetitive muscular activity that moves food through the intestine. How all these complex mechanisms are harmonized is still a mystery.
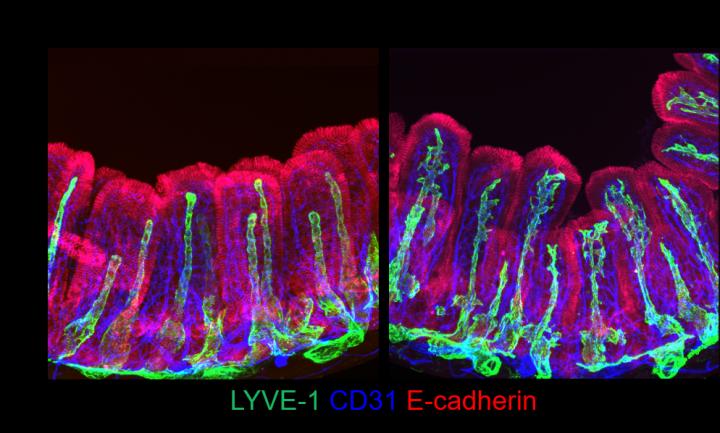
The research team was able to place a new piece towards completing this mysterious puzzle. The researchers found that the regulatory proteins YAP/TAZ in villi’s connective cells, the intestinal stromal cells, play a role in the growth of nearby lacteals. In mice with an abnormal hyperactivation of YAP/TAZ, the team observed atypical sprouting of lacteals and impaired dietary fat uptake.
“The lacteals in these mice looked like tridents, which is very intriguing, since we did not manipulate the lacteals themselves, but the surrounding cells,” says HONG Seon Pyo, first co-author of this study.
The researchers took a step further and discovered that intestinal stromal cells belong to several subtypes, with distinct gene expression and localizations within the villi. Among these subsets, three newly identified populations secrete VEGF-C – an essential molecule for lymphatic growth – upon YAP/TAZ activation. YANG Myung Jin, first co-author of this study, explains, “We were very surprised to see such heterogeneity in a cell population that was considered homogeneous.”
Lastly, researchers showed that mechanical force and osmotic stress regulate YAP/TAZ activity in stromal cells. In summary, mechanical stimulation activates YAP/TAZ in the intestinal stromal cells, which in turn release VEGF-C and can account for lacteal growth. CHO Hyunsoo, first co-author of this study notes, “This result implies a crucial link between the physiology of intestinal environment and biological interactions between cell types.”
“We are interested in investigating how each newly identified cell type works in healthy and diseased conditions,” adds Koh.
###
Media Contact
Dr. Seon Pyo Hong
[email protected]
Related Journal Article
http://dx.




