Tokyo, Japan – Researchers from Tokyo Metropolitan University have created sheets of transition metal chalcogenide “cubes” connected by chlorine atoms. While sheets of atoms have been widely studied e.g. graphene, the team’s work breaks new ground by using clusters instead. The team succeeded in forming nanoribbons inside carbon nanotubes for structural characterization, while also forming microscale sheets of cubes which could be exfoliated and probed. These were shown to be an excellent catalyst for generating hydrogen.
Credit: Tokyo Metropolitan University
Tokyo, Japan – Researchers from Tokyo Metropolitan University have created sheets of transition metal chalcogenide “cubes” connected by chlorine atoms. While sheets of atoms have been widely studied e.g. graphene, the team’s work breaks new ground by using clusters instead. The team succeeded in forming nanoribbons inside carbon nanotubes for structural characterization, while also forming microscale sheets of cubes which could be exfoliated and probed. These were shown to be an excellent catalyst for generating hydrogen.
Two-dimensional materials are a breakthrough in nanotechnology, realizing materials with exotic electronic and physical properties which are specific to their sheet-like nature. While graphene is well known, there has also been a lot of focus on transition metal chalcogenides (TMCs), composed of a transition metal and a group 16 element like sulfur or selenium. For example, nanosheets of TMCs have been shown to be able to emit light and show excellent performance as transistors.
But while advances are being made at a great pace, in most cases, it has been about getting atoms to form the right crystalline structure in sheet-like geometries. A team of researchers from Tokyo Metropolitan University led by Assistant Professor Yusuke Nakanishi was inspired to try a different approach: is it possible to use TMC clusters instead, and arrange them into two-dimensional patterns? This new route to assembling nanosheets would yield a whole different class of nanomaterials.
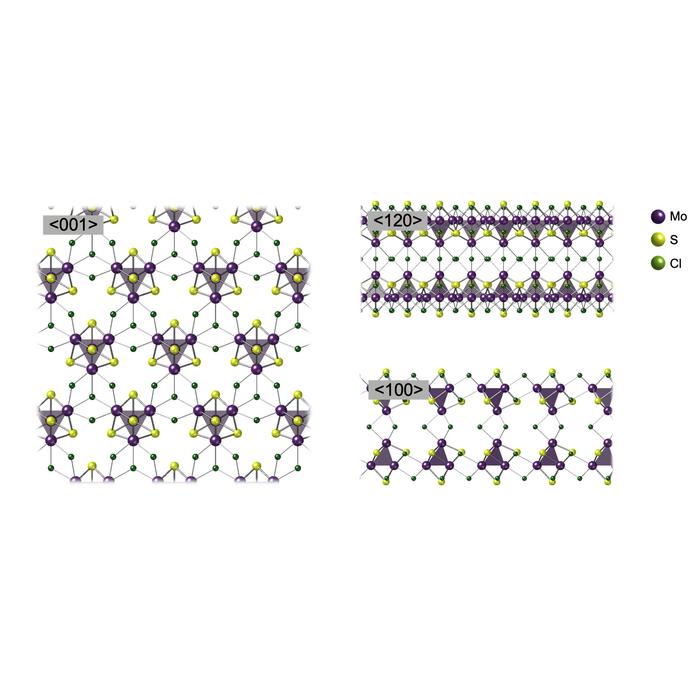
The team focused their efforts on cubic “superatomic” clusters of molybdenum and sulfur. They grew their material from a vapor of molybdenum (V) chloride and sulfur in the nanoscale confines of carbon nanotubes. The nanoribbons that are grown are well isolated and can be clearly imaged using transmission electron microscopy (TEM). They confirmed that their material consisted of isolated molybdenum sulfide “cubes” connected by chlorine atoms, distinct from cubic structures found in bulk materials.
But for the material to be useful in applications, it needs to be made in larger dimensions. In the same experiment, the team found a flaky material coating the inside of their glass reaction tube. By separating the solid from the walls, they discovered that it was made up of relatively large microscale flakes composed of the same superatomic clusters arranged in a hexagonal pattern. While the team have only begun to explore the potential of their new material, they have already shown theoretically that the same structure under tiny stresses could emit light. They also found that it might be an effective catalyst for the hydrogen evolution reaction (HER), most commonly seen when hydrogen is generated as a current passes through water. Compared with molybdenum disulfide, itself a promising catalytic material, the new layered material showed significantly higher current at lower voltages when probed, indicating greater efficiency.
While there’s more to come, their new approach to assembling nanosheets promises a whole range of new rationally designed materials with exciting new functions.
This work was supported by JSPS KAKENHI Grants from MEXT (Grant Numbers JP23H01807, JP24H00044, JP24K17708, JP24H01189, JP24H00478, JP22H05478, JP23H00277, JP21H05235, JP21K14484, JP21H05233, JP21H05232, JP21H05234, JP22H00283 and JP22H04957), and the PRESTO (Grant Number JPMJPR23H5), CREST (Grant Numbers JPMJCR20B1 and JPMJCR23A4), ACT-X (grant No. JPMJAX23DH), and FOREST (JPMJFR203K and JPMJFR213X) Programs from the JST.
#
Some of the figures have been adopted from the original paper, and modified.
Journal
Advanced Materials
DOI
10.1002/adma.202404249
Article Title
Superatomic layer of cubic Mo4S4 clusters connected by Cl cross-linking
Article Publication Date
25-Jul-2024




