The study was led by researchers at the National Cancer Institute (NCI)─designated Albert Einstein Cancer Center of Albert Einstein College of Medicine of Yeshiva University and Montefiore Einstein Center for Cancer Care and was published online June 03 in the Journal of the National Cancer Institute (JNCI).
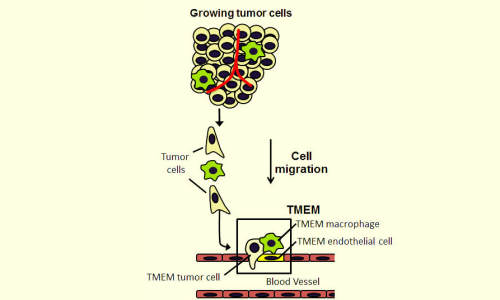
Metastasis requires the presence of three cells in direct contact on a blood vessel wall: a tumor cell that produces the protein MENA; a peri-vascular macrophage (cells that guide tumor cells to blood vessels); and a blood-vessel endothelial cell. The presence of three such cells in contact with each other is called a tumor microenvironment of metastasis, or TMEM, which is depicted within the box in this illustration. Photo Credit: Albert Einstein College of Medicine
“Tests assessing metastatic risk can help doctors identify which patients should receive aggressive therapy and which patients should be spared,” said Dr. Thomas Rohan, the lead and corresponding author of the study and professor and chair of epidemiology & population health at Einstein and Montefiore.
To measure the test’s effectiveness, the researchers used it on about 500 breast tumour specimens that had been collected over a 20-year period. The test proved more accurate in predicting the risk of distant tumour spread than a test closely resembling the leading breast cancer prognostic indicator on the market.
According to the NCI, 232,340 American women developed breast cancer last year and 39,620 women died from the disease. It is the most common cancer among women in the United States. Death from breast cancer is mainly due to distant metastasis, when cancer cells in the primary tumour invade blood vessels and travel via the bloodstream to form tumours elsewhere in the body.
Observing How Cancer Cells Metastasize
“Currently marketed tests assess risk for breast cancer metastasis by looking for changes in gene expression or in levels of proteins associated with growth of tumour cells,” said Dr. Joan Jones, senior author of the JNCI paper, professor of pathology of anatomy and structural biology and of epidemiology & population health at Einstein and attending pathologist at Montefiore Medical Center. “But those changes don’t reflect the mechanism by which individual tumour cells invade blood vessels, a necessary step for metastasis. By contrast, our test is based on what Einstein researchers learned from intravital imaging, which reveals biological processes deep within the tissues of a living animal. Using this technology, they determined how breast cancer tumour cells spread in rodents.”
Those observations showed that primary breast cancers metastasize when a specific trio of cells is present together in the same microanatomic site: an endothelial cell (a type of cell that lines the blood vessels), a perivascular macrophage (a type of immune cell found near blood vessels) and a tumour cell that produces high levels of Mena, a protein that enhances a cancer cell’s ability to spread. A site where these three cells touch is where tumour cells can enter blood vessels. That site is called a tumour microenvironment of metastasis, or TMEM (see accompanying figure).
Dr. Jones, who is also director of clinical imaging applications in Einstein’s Integrated Imaging Program (IIP), led a team of pathologists who applied the intravital imaging observations in living rodents to identify TMEMs in human breast biopsy specimens. The scientists developed a test that uses a triple immunostain containing antibodies to the three cell types that make up a TMEM. The test was then optimized using resources in the IIP, established with the support of benefactor Dr. Evelyn Gruss Lipper, an Einstein alumna (’71) and honorary Einstein Overseer.
The IIP combines the attributes of several different imaging technologies to reveal in great detail how cancer and other complex diseases get started and progress in the body, permitting the translation of basic-science observations into relevant clinical applications. The pioneering intravital imaging that made these discoveries possible was developed in the Gruss Lipper Biophotonics Center at Einstein.
Assessing Risk in Breast Cancer Patients
The JNCI paper describes results from a case-control study that evaluated tumour samples from a subset of women in the Kaiser Permanente Northwest health plan who were diagnosed with invasive ductal carcinoma of the breast between 1980 and 2000. TMEM testing was carried out on specimens from 259 women who later developed a distant metastasis (the cases) and on specimens from women who were alive and had not developed a distant metastasis (the controls). Controls were individually matched with cases so that women in each pair were the same age and were diagnosed with breast cancer in the same year. The pathology team applied the triple immunostain to specimens and counted the TMEMs. The team members did not know whether the specimens came from breast tumours that remained localized or from those that had metastasized to distant sites. The final TMEM score for each specimen was calculated by counting the total number of TMEMs observed within ten 400x magnification fields.
The TMEM test performed well at assessing metastatic risk for the study’s most populous cancer subgroup: women with oestrogen receptor positive/HER2- disease (i.e., their cancer cells possess oestrogen receptors but lack HER2 protein). Women with oestrogen receptor positive/HER2- disease account for about 60 percent of all cases of breast cancer. When women with this common type of breast cancer were divided into three groups based on the distribution of TMEM scores in the control group, the risk of distant metastasis turned out to be 2.7 times higher for women with tumours in the high-score TMEM group compared with women with tumours in the low-score TMEM group.
For comparison, all tumour specimens included in the study were also analyzed by the IHC4 test. This previously validated test assesses metastatic risk by measuring levels of several proteins in breast-tumour tissue. The IHC4 test is known to provide prognostic information comparable to the Oncotype DX test—a gene-expression test that is the most commonly used test for calculating metastatic risk in breast tumours.
Significant Findings
Overall, there was little agreement between the TMEM test and the IHC4 test regarding prediction of metastatic risk. As for assessing metastatic risk in the study’s most common type of breast cancer (ER+/HER2-), TMEM results were highly statistically significant while IHC4 scores were borderline significant at best. The findings confirmed results from a smaller study of the TMEM test (involving 30 pairs of tumour specimens) that was published by researchers from Einstein and other institutions in 2009.
“This assay could eventually reduce overtreatment of early stage breast cancer, which remains a major problem despite extensive use of other prognostic assays,” added study co-author Dr. Joseph Sparano, associate director for clinical research at the Albert Einstein Cancer Center, professor in the departments of medicine (oncology) and of obstetrics & gynecology and women’s health at Einstein and vice chair of medical oncology, Montefiore Einstein Center for Cancer Care.
“We’re pleased we found a strong and statistically significant association between TMEM score and risk of metastasis in the most common type of breast cancer,” said Dr. Rohan, who is also associate director for population sciences and leader of the cancer epidemiology program at Albert Einstein Cancer Center and the Harold and Muriel Block Chair in Epidemiology & Population Health at Einstein. “Further studies will certainly be needed to validate our test, but our findings suggest that it might prove useful for guiding treatment decisions in women with breast cancer.”
Story Source:
The above story is based on materials provided by Albert Einstein College of Medicine.