Osteoporosis is a skeletal condition that leads to the weakening of bones, making them porous, fragile, and prone to breakage. A whopping 8.9 million fractures are caused by osteoporosis annually, with one fracture occurring every three seconds! The aging population is the most vulnerable to primary osteoporosis, given, their frailty, and often, requires long-term therapy and support. Advances in healthcare and the corresponding rise in the aging population have put a strain on available resources, underscoring the need for effective therapies against osteoporosis.
Credit: Professor Tadayoshi Hayata from Tokyo University of Science
Osteoporosis is a skeletal condition that leads to the weakening of bones, making them porous, fragile, and prone to breakage. A whopping 8.9 million fractures are caused by osteoporosis annually, with one fracture occurring every three seconds! The aging population is the most vulnerable to primary osteoporosis, given, their frailty, and often, requires long-term therapy and support. Advances in healthcare and the corresponding rise in the aging population have put a strain on available resources, underscoring the need for effective therapies against osteoporosis.
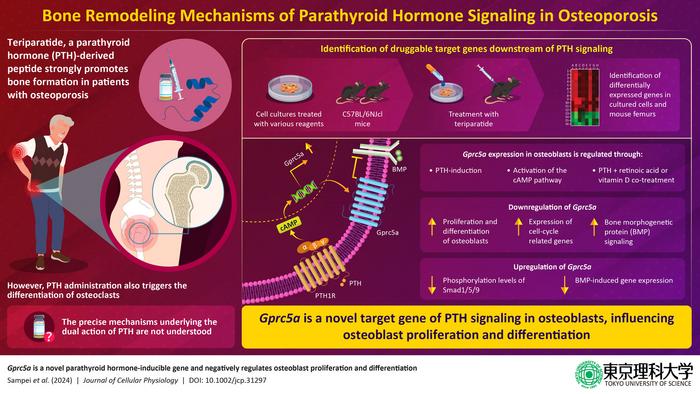
Induction of parathyroid hormone (PTH) signaling using the PTH-derived peptide – teriparatide, has demonstrated strong bone-promoting effects in patients with osteoporosis. These effects are mediated by osteogenesis, the process of bone formation involving the differentiation and maturation of bone-forming cells called osteoblasts. However, PTH induction is also associated with the differentiation of macrophages into osteoclasts, which are specialized cells responsible for bone resorption. Although, bone remodeling by osteoblasts and osteoclasts is crucial for maintaining skeletal health, PTH-induced osteoclast differentiation can decrease treatment efficacy in patients with osteoporosis. However, precise molecular mechanisms underlying the dual action of PTH signaling in bone remodeling are not well understood.
To bridge this gap, Professor Tadayoshi Hayata and Ms. Chisato Sampei, from Tokyo University of Science, along with their colleagues, conducted a series of experiments to identify druggable target genes downstream of PTH signaling in osteoblasts. Explaining the rationale behind their study published on 20 May, 2024, in the Journal of Cellular Physiology, corresponding author, Prof. Hayata says, “In Japan, it is estimated that 12.8 million people, or one in ten people, suffer from osteoporosis, which can significantly deteriorate their quality of life. Teriparatide is classified as a drug that promotes bone formation, but it also promotes bone resorption, which may limit bone formation. However, the full scope of its pharmacological action remains unknown.”
The researchers treated cultured mouse osteoblast cells and mice with teriparatide. They then assessed gene expression changes induced by PTH in both the cultured cells and bone cells isolated from the femurs of the treated animals, using advanced RNA-sequencing analysis. Among several upregulated genes, they identified a novel PTH-induced gene – ‘Gprc5a’, encoding an orphan G protein-coupled receptor, which has been previously explored as a therapeutic target. However, its precise role in osteoblast differentiation had not been fully understood.
PTH induction has been known to activate the cyclic adenosine monophosphate (cAMP) and protein kinase C (PKC) signaling pathways. Interestingly, the team found that in addition to PTH induction, activation of cAMP and PKC also resulted in overexpression of Gprc5a, albeit to a lesser extent, underscoring the potential involvement of other molecular pathways. Notably, upregulation of Gprc5a was suppressed upon inhibition of transcription, but, remained unaffected upon suppressing protein synthesis, suggesting that Gprc5a could be transcribed early on in response to PTH signaling and serves as a direct target gene.
Furthermore, the researchers examined the effect of Gprc5a downregulation on osteoblast proliferation and differentiation. Notably, while PTH induction alone did not affect cell proliferation, Gprc5a knockdown resulted in an increase in the expression of cell-cycle-related genes and osteoblast differentiation markers. These findings suggest that Gprc5a suppresses osteoblast proliferation and differentiation.
Diving deeper into the molecular mechanisms underlying the effects of Gprc5a, in PTH-induced osteogenesis, the researchers identified Activin receptor-like kinase 3 (ALK3) – a bone morphogenetic protein (BMP) signaling pathway receptor, as an interacting partner of Gprc5a. In line with their speculation, overexpression of Gprc5a indeed, led to suppression of BMP signaling via receptors including ALK3.
Overall, these findings reveal that Gprc5a – a novel inducible target gene of PTH, negatively regulates osteoblast proliferation and differentiation, by partially suppressing BMP signaling. Gprc5a can thus, be pursued as a novel therapeutic target while devising treatments against osteoporosis. The study sheds light on the complex process of bone remodeling and explains the bone-promoting and bone-resorbing effects of PTH signaling.
“Our study shows Gprc5a may function as a negative feedback factor for the bone formation promoting effect of teriparatide. Suppressing Gprc5a function may, therefore, increase the effectiveness of teriparatide in non-responding patients. In the future, we hope that our research will lead to improved quality of life and healthy longevity for people suffering from osteoporosis,” concludes Prof. Hayata.
We too hope that these findings pave the way for the development of effective therapies that can improve the lives of people living with osteoporosis.
***
Reference
DOI: https://doi.org/10.1002/jcp.31297
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Professor Tadayoshi Hayata from Tokyo University of Science
Tadayoshi Hayata became an Associate Professor and Principal Investigator at the Department of Molecular Pharmacology, Faculty of Pharmaceutical Science at the Tokyo University of Science in 2018. In 2023, he was promoted to Full Professor. His laboratory focuses on bone metabolism, cellular differentiation, molecular pharmacology, and similar fields to understand the nature of bone and joint diseases and find therapeutic targets. Prof. Hayata is affiliated with several Japanese Societies and the American Society for Bone and Mineral Research. He has published over 60 original articles and given over 150 presentations at academic conferences. In addition, his research on osteoporosis has made it to Japanese newspapers several times.
Funding information
This work was supported by JSPS KAKENHI Grant Numbers, JP25462287, JP25670639, JP26253085, JP18K09053, and JP21H03381
Journal
Journal of Cellular Physiology
DOI
10.1002/jcp.31297
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Gprc5a is a Novel Parathyroid Hormone-Inducible Gene and Negatively Regulates Osteoblast Proliferation and Differentiation
Article Publication Date
20-May-2024
COI Statement
The authors declare no conflict of interest.




