Researchers from Umeå have found a new way to synthesize graphene oxide which has significantly fewer defects compared to materials produced by most common method. Similarly good graphene oxide could be synthesized previously only using rather dangerous method involving extremely toxic fuming nitric acid.
Credit: Bartosz Gurzeda
Researchers from Umeå have found a new way to synthesize graphene oxide which has significantly fewer defects compared to materials produced by most common method. Similarly good graphene oxide could be synthesized previously only using rather dangerous method involving extremely toxic fuming nitric acid.
Graphene oxide is often used to produce graphene by removing oxygen. However, if you have holes in graphene oxide, you have holes also after converting it into graphene. Therefore, the quality of the graphene oxide is very important. Alexandr Talyzin and his research group at Umeå university in Sweden have now cracked the puzzle of how to safely make good graphene oxide. Their results were recently published in the scientific journal Carbon.
Graphene is often described as a “wonder material thanks to its flexibility, high mechanical strength and conductivity. But all properties of graphene are affected by defects. Graphene produced from graphene oxide has much worse than expected mechanical properties and conductivity.
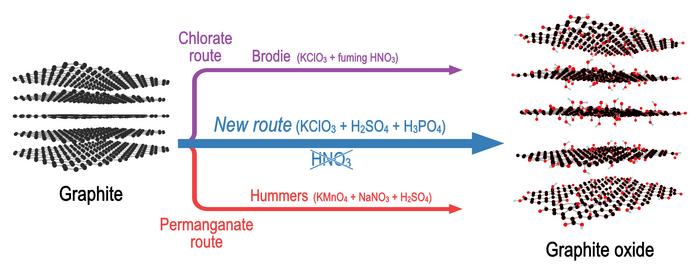
Many studies have demonstrated that synthesis by the most commonly used method, Hummers method, always results in a significant number of defects. The much older Brodie method provides nearly completely hole-free graphene oxide but this type of graphene oxide is still not produced by any companies and not available commercially.
“It is simply too dangerous and not suitable for industrial manufacturing,” says Alexandr Talyzin.
Now, researchers from Umeå have found a new method that combines the acid from the Hummers method (H2SO4) and the oxidant from the Brodie method (potassium chlorate), allowing them to produce graphene oxide with a number of defects as small as those in Brodie, but using a synthesis procedure as simple as Hummers oxidation.
“This method should be named after Bartosz Gurzeda, a researcher working in my group with the help of the Kempe Stipendium, as the Gurzeda method,” says Alexandr Talyzin.
According to Alexandr Talyzin, there are all reasons to believe that the Gurzeda method will become as popular as Hummers oxidation whenever defect-free graphene oxide is needed. This is for making graphene by removing oxygen groups or for the preparation of gas protection coatings, semi-permeable membranes, sensors, and many other possible applications.
In the recent decade, a lot of interest has also arisen for applications of graphene oxide itself. Layered graphene oxide materials are intensively studied for membrane applications with the dream of producing drinkable water by simple filtration of salts from sea water or creating semi-permeable protective coatings that allow water to pass while keeping dangerous organic pollutants, such as toluene, away.
“We want the research society to try and test this new graphene oxide in their applications and see the difference. Graphene oxide is not one material; it is a family of materials with rather different properties, providing us with infinite opportunities for new applications,” says Alexandr Talyzin.
Journal
Carbon
DOI
10.1016/j.carbon.2024.118899
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Graphite oxide by “chlorate route” oxidation without HNO3: Does acid matter?
Article Publication Date
9-Feb-2024




