Researchers led by Genki Kobayashi at the RIKEN Cluster for Pioneering Research in Japan have developed a solid electrolyte for transporting hydride ions (H−) at room temperature. This breakthrough means that the advantages of hydrogen-based solid-state batteries and fuel cells are within practical reach, including improved safety, efficiency, and energy density, which are essential for advancing towards a practical hydrogen-based energy economy.The study was published in the scientific journal Advanced Energy Materials.
Credit: RIKEN
Researchers led by Genki Kobayashi at the RIKEN Cluster for Pioneering Research in Japan have developed a solid electrolyte for transporting hydride ions (H−) at room temperature. This breakthrough means that the advantages of hydrogen-based solid-state batteries and fuel cells are within practical reach, including improved safety, efficiency, and energy density, which are essential for advancing towards a practical hydrogen-based energy economy.The study was published in the scientific journal Advanced Energy Materials.
For hydrogen-based energy storage and fuel to become more widespread, it needs to be safe, very efficient, and as simple as possible. Current hydrogen-based fuel cells used in electric cars work by allowing hydrogen protons to pass from one end of the fuel cell to the other through a polymer membrane when generating energy. Efficient, high-speed hydrogen movement in these fuel cells requires water, meaning that the membrane must be continually hydrated so that it does not dry out. This constraint adds an additional layer of complexity and cost to battery and fuel cell design that limits the practicality of a next-generation hydrogen-based energy economy. To overcome this problem, scientists have been struggling to find a way to conduct negative hydride ions through solid materials, particularly at room temperature.
The wait is over. “We have achieved a true milestone,” says Kobayashi. “Our result is the first demonstration of a hydride ion-conducting solid electrolyte at room temperature.”
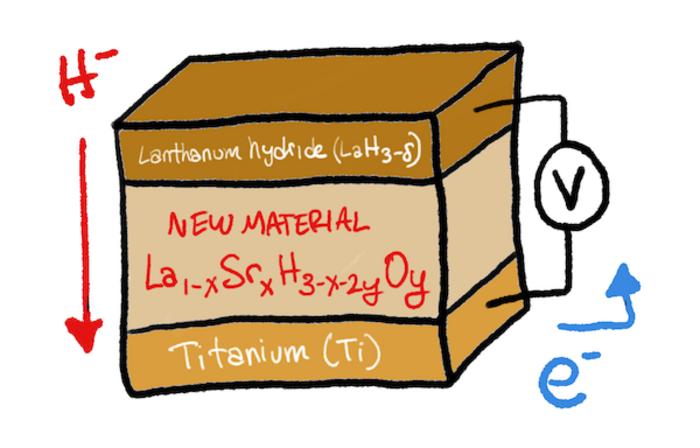
The team had been experimenting with lanthanum hydrides (LaH3-δ) for several reasons; the hydrogen can be released and captured relatively easily, hydride ion conduction is very high, they can work below 100°C, and have a crystal structure. But, at room temperature, the number of hydrogens attached to lanthanum fluctuates between 2 and 3, making it impossible to have efficient conduction. This problem is called hydrogen non-stoichiometry, and was the biggest obstacle overcome in the new study. When the researchers replaced some of the lanthanum with strontium (Sr) and added just a pinch of oxygen—for a basic formula of La1-xSrxH3-x-2yOy, they got the results they were hoping for.
The team prepared crystalline samples of the material using a process called ball-milling, followed by annealing. They studied the samples at room temperature and found that they could conduct hydride ions at a high rate. Then, they tested its performance in a solid-state fuel cell made from the new material and titanium, varying the amounts of strontium and oxygen in the formula. With an optimal value of at least 0.2 strontium, they observed complete 100% conversion of titanium to titanium hydride, or TiH2. This means that almost zero hydride ions were wasted.
“In the short-term, our results provide material design guidelines for hydride ion-conducting solid electrolytes,” says Kobayashi. “In the long-term, we believe this is an inflection point in the development of batteries, fuel cells, and electrolytic cells that operate by using hydrogen.” The next step will be to improve performance and create electrode materials that can reversibly absorb and release hydrogen. This would allow batteries to be recharged, as well as make it possible to place hydrogen in storage and easily release it when needed, which is a requirement for hydrogen-based energy use.
Journal
Advanced Energy Materials
DOI
10.1002/aenm.202301993




