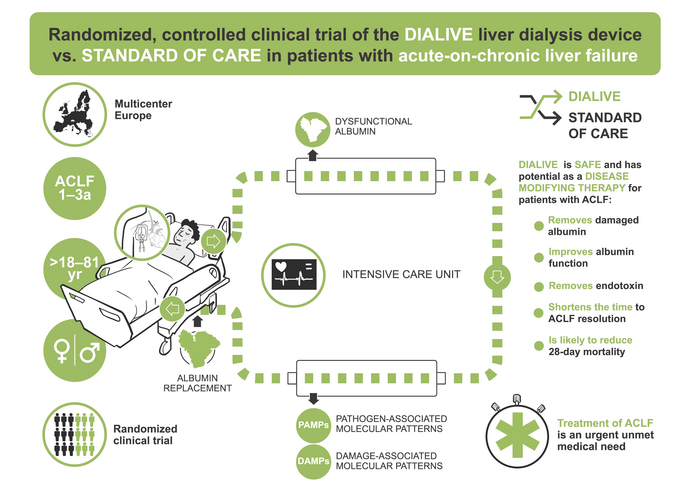
Amsterdam, June 1, 2023 – Acute-on-chronic liver failure (ACLF) occurs in 30% of hospitalized cirrhosis patients, leading to over one million deaths worldwide each year. Currently the only potential treatment for this condition is liver transplantation, which is available to very few patients. A first-in-human randomized controlled clinical trial using DIALIVE, a novel liver dialysis device, demonstrated its potential as a disease-modifying therapy and resolved liver failure significantly faster and in a greater proportion of patients compared with controls. Treatment of ACLF patients resulted in significant improvements in all the main underlying pathophysiological mechanisms of ACLF. Results appear in the Journal of Hepatology, published by Elsevier.
Credit: Journal of Hepatology
Amsterdam, June 1, 2023 – Acute-on-chronic liver failure (ACLF) occurs in 30% of hospitalized cirrhosis patients, leading to over one million deaths worldwide each year. Currently the only potential treatment for this condition is liver transplantation, which is available to very few patients. A first-in-human randomized controlled clinical trial using DIALIVE, a novel liver dialysis device, demonstrated its potential as a disease-modifying therapy and resolved liver failure significantly faster and in a greater proportion of patients compared with controls. Treatment of ACLF patients resulted in significant improvements in all the main underlying pathophysiological mechanisms of ACLF. Results appear in the Journal of Hepatology, published by Elsevier.
“DIALIVE is a novel liver dialysis device invented at University College London (UCL) by Dr. Nathan Davies and me. It is based on an understanding of the mechanisms underlying ACLF, which are what we are correcting with this machine by exchanging dysfunctional albumin and removing damage- and pathogen-associated molecular patterns,” explained lead investigator Rajiv Jalan, PhD, UCL Institute for Liver & Digestive Health, based at the Royal Free Hospital, London, and European Foundation for the Study of Chronic Liver Failure (EF Clif), Barcelona. “Two previous preclinical trials showed that this device saved the lives of animals with liver failure. These data were used to apply for an EU grant (ALIVER) to conduct this clinical trial.”
In this multicenter, randomized clinical trial investigators evaluated 32 alcoholic cirrhosis patients with ACLF who were treated with either DIALIVE or standard of care for up to five days. End points were assessed at Day 10. The primary aim was to evaluate DIALIVE’s safety in acute ACLF patients, while secondary aims were to assess clinical effects, device performance and effect on pathophysiologically-relevant biomarkers. Safety was assessed in all patients. The secondary aims were assessed in a pre-specified subgroup of 30 patients who had at least three treatment sessions with DIALIVE.
The study, which involved multiple centers across Europe, was led by clinical investigator Banwari Agarwal, MBBS, MD, consultant in liver ICU at the Royal Free Hospital.
It confirmed that the DIALIVE system is safe. In addition, patients experienced significant improvements on all the main underlying pathophysiological mechanisms of ACLF. This included improvement in albumin function, reduction in products of cell death and endotoxin, restoration of endothelial function, and cell signaling. Although the study was not powered for efficacy, it showed that the proportion of patients in which ACLF was resolved was greater with DIALIVE, and the time to resolution of ACLF was significantly reduced. It did not, however, reduce mortality in this small study. Therefore, larger clinical trials are needed in future to confirm DIALIVE’s efficacy and establish its safety.
“This is the first treatment that has shown such a rapid clinical effect in patients with ACLF,” said co-lead investigator Steffen Mitzner, MD, PhD, Fraunhofer IZI and Department of Medicine II, Division of Gastroenterology and Endocrinology, Rostock University Medical Center, Rostock, Germany. “The patients that resolved ACLF remained free of ACLF for 28 days despite only being treated with the device for three days. The improvement in biomarkers underlying the pathophysiology of ACLF was sustained even five days after stopping therapy.”
“These data indicate that DIALIVE may be a disease-modifying therapy for ACLF patients and could impact the outcome of patients with ACLF, for which there is no available therapy except liver transplantation available to a very small minority of these patients,” concluded Dr. Jalan. “The potential impact is huge if these data can be confirmed in larger clinical trials.”
“The positive results from this trial are great news for ACLF patients, and we are now preparing for a larger clinical trial,” added Carrie Morgan, Vice President, Clinical Operations, Yaqrit Discovery Ltd, a UCL spin-off company located in London and producer of DIALIVE.
ACLF is characterized clinically by multiorgan failure and high risk of short-term mortality and pathophysiologically by the presence of systemic inflammation. ACLF is a major global problem with prevalence rates of 20% to 30% in hospitalized cirrhosis patients. The worldwide reported mortality rate according to the EASL-CLIF Consortium definition is between 30% to 50% at 28-days from presentation.
Journal
Journal of Hepatology
DOI
10.1016/j.jhep.2023.03.013
Method of Research
Randomized controlled/clinical trial
Subject of Research
People
Article Title
Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on-chronic liver failure
Article Publication Date
1-Jun-2023



