In a significant leap forward in cancer biology, researchers from the Institute of Industrial Science at The University of Tokyo, in collaboration with Kanazawa University, Institute of Science Tokyo, and Kyorin University School of Medicine, have uncovered a detailed mechanism illustrating how clusters of tumor cells breach blood vessel walls to enter the bloodstream. This groundbreaking study, recently published in the journal iScience, elucidates the elusive process of tumor cell cluster intravasation, a crucial step facilitating metastasis throughout the body. Metastasis remains the principal cause of cancer mortality, and understanding its underlying cellular behaviors promises new therapeutic avenues.
The complexity of solid tumors stems from their heterogeneous population of cells, often numbering in the millions, which poses an ongoing challenge to treatment strategies. Surgical excision or systemic therapies may be effective in controlling primary tumors, yet metastatic dissemination significantly worsens patient prognosis. The researchers focused on circulating tumor cell (CTC) clusters—cohesive groups of cells detaching from the primary neoplasm and found within patient bloodstream samples. These clusters, in contrast to singular tumor cells, exhibit enhanced metastatic potential, yet the molecular and biophysical pathways facilitating their intravasation—penetration into blood vessels—have eluded the scientific community until now.
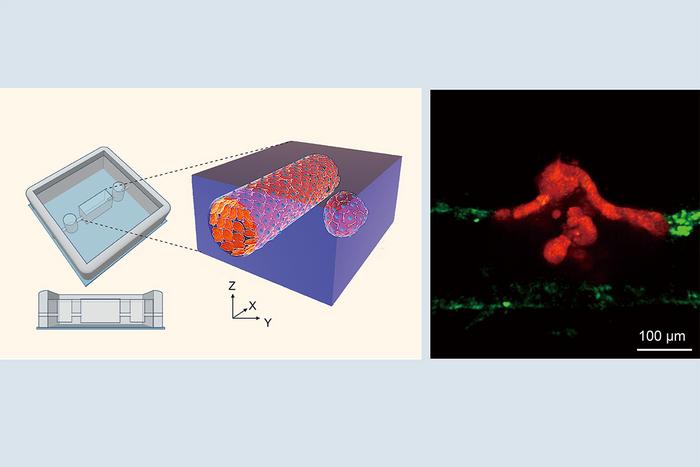
Addressing this gap, the interdisciplinary team engineered an advanced three-dimensional in vitro model mimicking the tumor microenvironment with unprecedented precision. This “tumor-microvessel on-a-chip” system integrates artificial blood vessels alongside intestinal tumor organoids derived from neoplastic tissue. By carefully manipulating the spatial orientation of these organoids relative to the vessel walls, researchers gained the ability to visualize, in real time, dynamic interactions between tumor cell clusters and the vascular endothelium using high-resolution live-imaging techniques.
What emerged from these observations was a striking phenomenon. Tumor cell clusters displayed directed migration toward the vascular structures, eventually disrupting the integrity of vessel walls to intravasate. Upon entering the lumen of the blood vessel, these cohesive clusters dispersed—likely a prelude to dissemination and colonization at distant sites. The vessel walls themselves revealed molecular transformations indicative of an endothelial-to-mesenchymal transition (EndMT), a cellular reprogramming where endothelial cells lose their characteristic features and adopt a mesenchymal, more malleable state.
Central to this transition was the upregulation of key cytokines, particularly transforming growth factor-beta (TGF-β) and activin, secreted in response to tumor cell cluster proximity. These molecules orchestrate complex signaling cascades that compromise the endothelial barrier function, rendering the vessel walls more permissive to cluster infiltration. Such molecular insight clarifies how tumor clusters effectively “take over” segments of the blood vessel wall to penetrate the bloodstream, a process previously only hypothesized without direct mechanistic evidence.
This discovery holds significant implications for therapeutic development. Preventing or mitigating the intravasation of CTC clusters could curb metastatic spread and improve patient survival rates. The novel 3D in vitro system offers a versatile platform for screening anti-metastatic compounds aimed specifically at interrupting the signaling pathways or mechanical disruptions facilitating vascular entry. Future research leveraging this technology may reveal targeted molecular inhibitors that reinforce endothelial barrier integrity against malignant infiltration.
Moreover, the study underscores the importance of tumor architecture and microenvironmental factors in metastasis. While isolated tumor cells possess metastatic capability, the collective behavior of clusters endows them with unique advantages, such as enhanced survival in circulation and evasion of immune surveillance. By visibly capturing the process of cluster migration, vascular wall disruption, and luminal dissemination within a controlled laboratory setting, the research provides a vital window into the physical and biochemical interplay driving metastatic progression.
Yukinori Ikeda and Makoto Kondo, co-lead authors of the study, emphasize the novelty of their observations: the direct interaction between compact cell groups and vascular endothelial cells, culminating in site-specific partial endothelial disruption followed by cluster dispersal. This process defies traditional views that cancer cells primarily migrate individually, reaffirming that collective cell dynamics are crucial to understanding tumor biology comprehensively.
Senior author Professor Yukiko Matsunaga reflects on the translational potential of these findings, suggesting that clinical strategies focusing on interrupting cluster-mediated metastasis may transform treatment paradigms in advanced cancers. These could include localized delivery of TGF-β inhibitors, enhancement of endothelial junction proteins, or novel biomaterials designed to strengthen vessel walls against tumor-induced breakdown.
Importantly, this study demonstrates the power of organ-on-a-chip technology as an investigative tool. By faithfully reproducing aspects of tumor-vessel interfaces, researchers can dissect cellular behaviors that remain inaccessible within living organisms due to complexity and the invasiveness of traditional sampling. Such systems herald a new era of cancer research where microengineering and cell biology merge to unravel intricate disease mechanisms.
In summary, by unveiling the specific steps and molecular drivers enabling tumor cell clusters to hijack blood vessels and access the circulatory system, this research opens promising pathways toward innovative interventions against cancer metastasis. As metastasis underlies the majority of cancer-related deaths, targeted approaches inspired by these discoveries may dramatically reshape prognostic outlooks for patients worldwide.
Subject of Research: Mechanisms of tumor cell cluster intravasation and metastasis using a 3D tumor-microvessel on-a-chip model.
Article Title: A tumor-microvessel on-a-chip reveals a mechanism for cancer cell cluster intravasation
News Publication Date: 19-May-2025
Web References:
https://doi.org/10.1016/j.isci.2025.112517
Image Credits: Institute of Industrial Science, The University of Tokyo
Keywords: Cell biology, Cancer cells, Blood vessels, Vascular cells, Circulating tumor cells, Endothelial cells
Tags: biophysical behaviors of tumor cellsblood vessel breach by tumorscancer biology research breakthroughscirculating tumor cell clustersin vitro cancer modelinterdisciplinary cancer research collaborationmetastasis and cancer mortalitymolecular pathways in cancernew cancer treatment insightssolid tumor heterogeneitytherapeutic strategies for metastasistumor cell intravasation mechanism