Researchers from the group of Eva van Rooij used advanced sequencing technology to better understand the heart disease arrhythmogenic cardiomyopathy, in which heart muscle tissue is replaced by fat cells. Using explanted human hearts, they found regions in which heart muscle was actively degenerated and identified a new gene, ZBTB11, that drives heart muscle cell degradation. The results were published in Cardiovascular Research on 17 May 2022.
Credit: GPA Lacraz, copyright Hubrecht Institute
Researchers from the group of Eva van Rooij used advanced sequencing technology to better understand the heart disease arrhythmogenic cardiomyopathy, in which heart muscle tissue is replaced by fat cells. Using explanted human hearts, they found regions in which heart muscle was actively degenerated and identified a new gene, ZBTB11, that drives heart muscle cell degradation. The results were published in Cardiovascular Research on 17 May 2022.
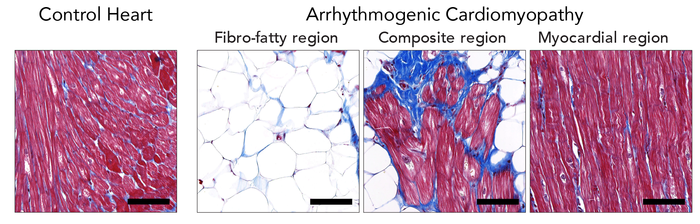
Arrhythmogenic cardiomyopathy (ACM) is a familial heart disease in which heart muscle tissue is replaced by fat cells. It can lead to life threatening, irregular heartbeats. Currently, no therapy exists to cure arrhythmogenic cardiomyopathy and patients may ultimately need a heart transplantation. Therefore, the group of Eva van Rooij at the Hubrecht Institute collaborated with the UMC Utrecht with the aim to better understand the process of heart muscle degeneration in ACM, to ultimately contribute to the identification of new therapeutic targets to treat the disease.
Local differences
Previous studies on ACM used methods that take a snapshot of the gene expression in diseased tissue to aim at understanding what happens in the disease, but lacked the spatial resolution that is required to identify local differences within the heart. Therefore, the group of Eva van Rooij collaborated with the group of Alexander van Oudenaarden to use a technique called Tomo-Seq, that enabled the researchers to study gene expression within different areas of the heart. It turned out that knowing the location of the cells with changed gene activity was key to the discovery of novel areas of heart muscle degeneration.
Human hearts
The researchers used explanted hearts from ACM patients that received a heart transplantation. Heart disease is often studied in cultured cells or animal models, since the use of patient heart tissue is usually not possible. However, due to a collaboration with Aryan Vink from the Pathology Department of UMC Utrecht, the researchers had access to one of the largest and best documented heart tissue biobanks of the world. This gave them the unique opportunity to study the disease process in patient material. The researchers were able to closely look at the patient heart muscle cells that are degenerating, and identified new genes that may play an important role in this process. Among these genes is the transcription factor ZBTB11, a gene that was not previously known to be involved in arrhythmogenic cardiomyopathy.
Fat cells
The gene ZBTB11 is specifically expressed in the heart muscle cells that are close to the fat cells. The researchers showed that the activity of ZBTB11 induced degeneration of heart muscle cells, a process that is key in arrhythmogenic cardiomyopathy. This indicates that the fatty regions in the heart induce damage to the neighboring heart muscle cells. In the future, the researchers aim to study if heart muscle damage can be slowed down or even reversed in arrhythmogenic cardiomyopathy.
~~~
Publication
Spatial transcriptomics unveils ZBTB11 as a regulator of cardiomyocyte degeneration in arrhythmogenic cardiomyopathy. Cornelis J. Boogerd, Grégory P.A. Lacraz, Ábel Vértesy, Sebastiaan J. van Kampen, Ilaria Perini, Hesther de Ruiter, Danielle Versteeg, Andreas Brodehl, Petra van der Kraak, Mauro Giacca, Nicolaas de Jonge, Jan Philipp Junker, Alexander van Oudenaarden, Aryan Vink, Eva van Rooij. Cardiovascular Research 2022.
Eva van Rooij is group leader at the Hubrecht Institute and professor of Molecular Cardiology at the University Medical Center Utrecht.
About the Hubrecht Institute
The Hubrecht Institute is a research institute focused on developmental and stem cell biology. It encompasses 19 research groups that perform fundamental and multidisciplinary research, both in healthy systems and disease models. The Hubrecht Institute is a research institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), situated on Utrecht Science Park. Since 2008, the institute is affiliated with the UMC Utrecht, advancing the translation of research to the clinic. The Hubrecht Institute has a partnership with the European Molecular Biology Laboratory (EMBL). For more information, visit http://www.hubrecht.eu.
Journal
Cardiovascular Research
DOI
10.1093/cvr/cvac072
Method of Research
Observational study
Subject of Research
Human tissue samples
Article Title
Spatial transcriptomics unveils ZBTB11 as a regulator of cardiomyocyte degeneration in arrhythmogenic cardiomyopathy
Article Publication Date
16-May-2022




