Unprecedented views of the interior of cells and other nanoscale structures are now possible thanks to innovations in expansion microscopy. The advancements could help provide future insight into neuroscience, pathology, and many other biological and medical fields.
Credit: Courtesy of Carnegie Mellon University
Unprecedented views of the interior of cells and other nanoscale structures are now possible thanks to innovations in expansion microscopy. The advancements could help provide future insight into neuroscience, pathology, and many other biological and medical fields.
In the paper “Magnify is a universal molecular anchoring strategy for expansion microscopy,” published Jan. 2 in the journal Nature Biotechnology, collaborators from Carnegie Mellon University, the University of Pittsburgh and Brown University describe new protocols for dubbed Magnify.
“Magnify can be a potent and accessible tool for the biotechnology community,” said Yongxin (Leon) Zhao, the Eberly Family Career Development Associate Professor of Biological Sciences.
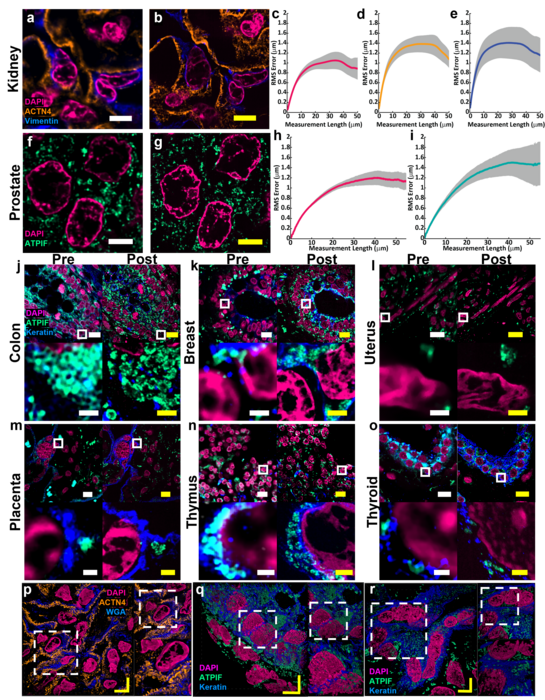
Zhao’s Biophotonics Lab is a leader in the field of enabling super-resolution imaging of biological samples through physically expanding samples in a process known as expansion microscopy. Through the process, samples are embedded in a swellable hydrogel that homogenously expands to increase the distance between molecules allowing them to be observed in greater resolution. This allows nanoscale biological structures that previously only could be viewed using expensive high-resolution imaging techniques to be seen with standard microscopy tools.
Magnify is a variant of expansion microscopy that allows researchers to use a new hydrogel formula, invented by Zhao’s team, that retains a spectrum of biomolecules, offers a broader application to a variety of tissues, and increases the expansion rate up to 11 times linearly or ~1,300 folds of the original volume.
“We overcame some of the longstanding challenges of expansion microscopy,” Zhao said. “One of the main selling points for Magnify is the universal strategy to keep the tissue’s biomolecules, including proteins, nucleus snippets and carbohydrates, within the expanded sample.”
Zhao said that keeping different biological components intact matters because previous protocols required eliminating many various biomolecules that held tissues together. But these molecules could contain valuable information for researchers.
“In the past, to make cells really expandable, you need to use enzymes to digest proteins, so in the end, you had an empty gel with labels that indicate the location of the protein of interest,” he said. With the new method, the molecules are kept intact, and multiple types of biomolecules can be labeled in a single sample.
“Before, it was like having single-choice questions. If you want to label proteins, that would be the version one protocol. If you want to label nuclei, then that would be a different version,” Zhao said. “If you wanted to do simultaneous imaging, it was difficult. Now with Magnify, you can pick multiple items to label, such as proteins, lipids and carbohydrates, and image them together.”
Lab researchers Aleksandra Klimas, a postdoctoral researcher and Brendan Gallagher, a doctoral student, were first co-authors on the paper.
“This is an accessible way to image specimens in high resolution,” Klimas said. “Traditionally, you need expensive equipment and specific reagents and training. However, this method is broadly applicable to many types of sample preparations and can be viewed with standard microscopes that you would have in a biology laboratory.”
Gallagher, who has a background in neuroscience, said their goal was to make the protocols as compatible as possible for researchers who could benefit from adopting the Magnify as part of their tool kits.
“One of the key concepts that we tried to keep in mind was to meet researchers where they are and have them change as few things in their protocols as possible,” Gallagher said. “It works with different tissue types, fixation methods and even tissue that has been preserved and stored. It is very flexible, in that you don’t necessarily need to redesign experiments with Magnify in mind completely; it will work with what you have already.”
For researchers such as Simon Watkins, the founder and director of the Center for Biologic Imaging at the University of Pittsburgh and the Pittsburgh Cancer Institute, the fact that the new protocol is compatible with a broad range of tissue types — including preserved tissue sections — is important. For example, most expansion microscopy methods are optimized for brain tissue. In contrast, Magnify was tested on samples from various human organs and corresponding tumors including breast, brain and colon.
“Let’s say you have a tissue with dense and non-dense components, this gets around tissues that previously wouldn’t expand isometrically,” Watkins said. “Leon has been working hard on this to make this protocol work with tissues that have been archived.”
Xi (Charlie) Ren, an assistant professor of biomedical engineering at Carnegie Mellon, studies the lung tissue and how to model its morphogenesis and pathogenesis. Part of his research involves researching the motile cilia that function to clear mucus in the human conducting airway. At 200 nanometers in diameter and just a few micrometers in length, the structures are too small to see without time-intensive technology such as electron microscopy. Working in collaboration with Zhao’s lab, Ren’s team developed and delivered lung organoid models with specific defects in cilia ultrastructure and function to validate the ability of Magnify to visualize clinically relevant cilia pathology.
“With the latest Magnify techniques, we can expand those lung tissues and start to see some ultrastructure of the motile cilia even with a regular microscope, and this will expedite both basic and clinical investigations” he said.
The researchers also were able to view defects in cilia in patient-specific lung cells known to have genetic mutations.
“The lung tissue engineering community always needs a better way to characterize the tissue system that we work with,” Ren said. He added that this work is an important first step and he hopes the collaborative work with Zhao’s lab will further be refined and applied to pathology samples found in tissue banks.
Finally, the hydrogel used in Magnify and developed in the Zhao lab is more robust than its predecessor, which was very fragile, causing breaks during the process.
“We are hoping to develop this technology to make it more accessible to the community,” he said. “There are different directions this can go. There’s a lot of interest in using this kind of tissue expansion technology for basic science.”
Alison Barth, the Maxwell H. and Gloria C. Connan Professor in the Life Sciences at Carnegie Mellon, studies synaptic connectivity during learning. She said the broad applications provided by the new methods will be a boon for researchers.
“The brain is a great place to take advantage of these super-resolution techniques,” said Barth, who collaborates with the Zhao Lab on several studies. “Microscopy methods will be beneficial for synaptic phenotyping and analysis across different brain conditions.
“One of the major advances in this paper is the method’s ability to work on many different types of tissue specimens.”
Additional study authors include Piyumi Wijesekara, Emma F. DiBernardo, Zhangyu Cheng of Carnegie Mellon; Sinda Fekir and Christopher I. Moore of Brown University; Donna B. Stolz of Pitt; Franca Cambi of Pitt and Veterans Administration; and Steven L Brody and Amjad Horani of Washington University.
This work was supported by Carnegie Mellon, the Kaufman Foundation, and the DSF Charitable Foundation, U.S. Department of Defense (VR190139), the National Institutes of Health (DP2 OD025926-01 and NIH RF1 MH114103), Air Force Office of Scientific Research (FA9550-19-1-13022629), NeuroNex (GR5260228.1001) and Brown University.
Journal
Nature Biotechnology
DOI
10.1038/s41587-022-01546-1
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Magnify is a universal molecular anchoring strategy for expansion microscopy
Article Publication Date
2-Jan-2023
COI Statement
The authors declare the following competing financial interest(s): Y.Z. and A.K. are inventors on several inventions related to ExM.




