Unlocking the atmosphere’s big mystery
Credit: UCI School of Biological Sciences
A key mystery about the gas comprising most of our atmosphere is closer to being solved following a discovery by University of California, Irvine biologists. Their findings are the first step in understanding the biological mechanism for breaking down nitrogen gas. Besides yielding groundbreaking knowledge, the information holds promise for developing environmentally friendly and cheaper ways to make products such as fertilizer and fuel. The team’s research has just been published in the journal Science.
Activation of the nitrogen gas (N2), which composes 78% of the atmosphere, has long stymied scientists. “The strong triple-bond between the nitrogen atoms in N2 makes this compound difficult to break apart, and thus nearly inert.” said Molecular Biology and Biochemistry Chancellor’s Professor Markus Ribbe. “Researchers have worked for decades to fully understand how nature can activate the nitrogen gas and break it down for biological purposes.”
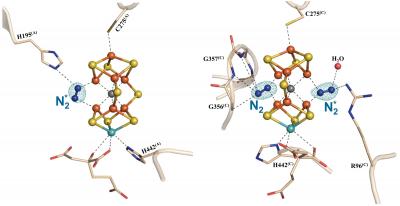
However, the teams of Professors Yilin Hu and Markus Ribbe, from the department of moleculary biology and biochemistry, have discovered how the enzyme nitrogenase can bind to N2 as the initial step towards its activation. X-ray crystallographic analysis showed that the three sulfur sites at the “belt region” of the FeMo cofactor in the active site of the enzyme are labile during catalysis, with the cofactor in one subunit of the enzyme having one, and the other subunit of the enzyme having two, of the three belt sulfur atoms replaced by distinct nitrogen species during the binding and reduction of N2. These findings are entirely unexpected and shed light on the sparsely understood mechanism of N2 reduction, pointing to a key role of belt sulfur displacement in proper nitrogenase function.
“We are optimistic that with further research, we will be able to demonstrate how this entire mechanism works,” said Professor Hu.
In addition to revealing important scientific insights, the discovery could ultimately transform manufacturing. Because this natural process is poorly understood, industries turn nitrogen gas into commercial products through other methods that take an environmental toll. For example, the most common procedure for breaking down N2 to produce ammonia fertilizer for agriculture, called the Haber-Bosch process, relies on very high heat and pressure.
Professor Ribbe said: “Once we understand how nature activates nitrogen gas under ambient conditions, it opens the way for developing manufacturing processes that use less energy and are also cheaper.” In addition to fertilizer, the discovery could have implications for alternative fuel production. Professors Hu and Ribbe, who have focused much of their research on nitrogenase over the years, have already discovered that the enzyme can convert carbon dioxide and carbon monoxide into hydrocarbons, which are major components in carbon fuels.
###
Collaborating closely with Professors Ribbe and Hu on the project were Wonchull Kang, Chi Chung Lee and Andrew Jasniewski from UCI. Funding for the study was provided by the U. S. Department of Energy – Basic Energy Sciences (BES) and the National Institutes of Health.
About the University of California, Irvine: Founded in 1965, UCI is the youngest member of the prestigious Association of American Universities. The campus has produced three Nobel laureates and is known for its academic achievement, premier research, innovation and anteater mascot. Led by Chancellor Howard Gillman, UCI has more than 36,000 students and offers 222 degree programs. It’s located in one of the world’s safest and most economically vibrant communities and is Orange County’s second-largest employer, contributing $5 billion annually to the local economy. For more on UCI, visit http://www.
Media Contact
Rahasson Ager
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




