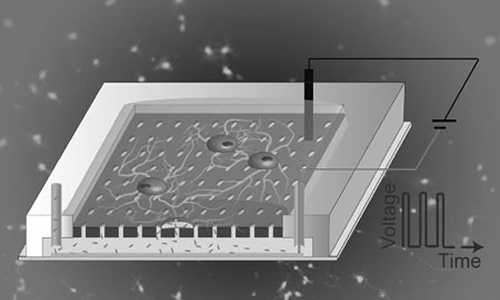
Electroporation is a powerful technique in molecular biology. By using an electrical pulse to create a temporary nanopore in a cell membrane, researchers can deliver chemicals, drugs, and DNA directly into a single cell.
But existing electroporation methods require high electric field strengths and for cells to be suspended in solution, which disrupts cellular pathways and creates a harsh environment for sensitive primary cells. This makes it nearly impossible for researchers to study the cells naturally, in a setting that encourages the cells to continue differentiating and expanding.
A Northwestern University collaboration has developed a novel microfluidic device that allows for electroporation of stem cells during differentiation, making it possible to deliver molecules during this pivotal time in a cell’s life. This provides the conditions needed to study primary cells, such as neurons, opening doors for exploration of the pathogenic mechanisms of neural diseases and potentially leading to new gene therapies.
Developed by Horacio Espinosa, the James and Nancy Farley Professor of Manufacturing and Entrepreneurship at the McCormick School of Engineering, and John Kessler, the Ken and Ruth Davee Professor of Stem Cell Biology at the Feinberg School of Medicine, the localized electroporation device (LEPD) can be applied to adherent cells, which are grown on an artificial substrate as opposed to free-floating in a culture medium and are able to continue growing and differentiating.
“The ability to deliver molecules into adherent cells without disrupting differentiation is needed for biotechnology researchers to advance both fundamental knowledge and the state-of-the-art in stem cell research,” Espinosa said.
“Non-destructive manipulation of cells over time and in the correct environment is a key enabling technology highly needed within the biology and medical research communities,” Kessler said.
Supported by the National Science Foundation and the National Institutes of Health, the research is described in a paper published in the September 10 issue of Lab on a Chip, the journal of The Royal Society of Chemistry, and was also highlighted on the journal’s back cover. Other authors on the paper include Wonmo Kang, Juan P. Giraldo-Vela, Shiva Nathamgari, Tammy McGuire, and Rebecca McNaughton.
The team fabricated the LEPD by employing a commonly used polymer for rapid prototyping of microfluidic devices for biological applications. It consists of circulation microchannels beneath a cell culture chamber made up of a perforated substrate and built-in electrodes. Although the main applications of the initial research examined neurons, the device is a general tool that can be used for any type of adherent cell.
Story Source:
The above story is based on materials provided by Northwestern University.