Credit: Oak Ridge National Laboratory
A new class of drugs that combat antibiotic resistance has been discovered by a University of Oklahoma researcher and team. In the study supported by the National Institutes of Health, laboratory experiments were combined with supercomputing modeling to identify molecules that boost the effect of antibiotics on disease-causing bacteria.
Helen Zgurskaya, professor of chemistry and biochemistry in the OU College of Arts and Sciences, and OU team members Narges Abdali, Julie Chaney, David Wolloscheck and Valentin Rybenkov, collaborated with Jeremy Smith, Jerry Parks and Jerome Baudry, the University of Tennessee-Oak Ridge National Laboratory Center for Molecular Biophysics; Adam Green, UT; and Keith Haynes and John Walker, Saint Louis University School of Medicine. They collectively identified four new chemicals that seek out and disrupt bacterial proteins called "efflux pumps", a major cause of antibiotic resistance in bacteria.
"The supercomputing power of ORNL's Titan supercomputer allowed us to perform large-scale simulations of the drug targets and to screen many potential compounds quickly," said Zgurskaya, head of the OU Antibiotic Discovery and Resistance Group at the Stephenson Life Sciences Research Center. "The information we received was combined with our experiments to select molecules that were found to work well, and this should drastically reduce the time needed to move from the experimental phase to clinical trials," she added.
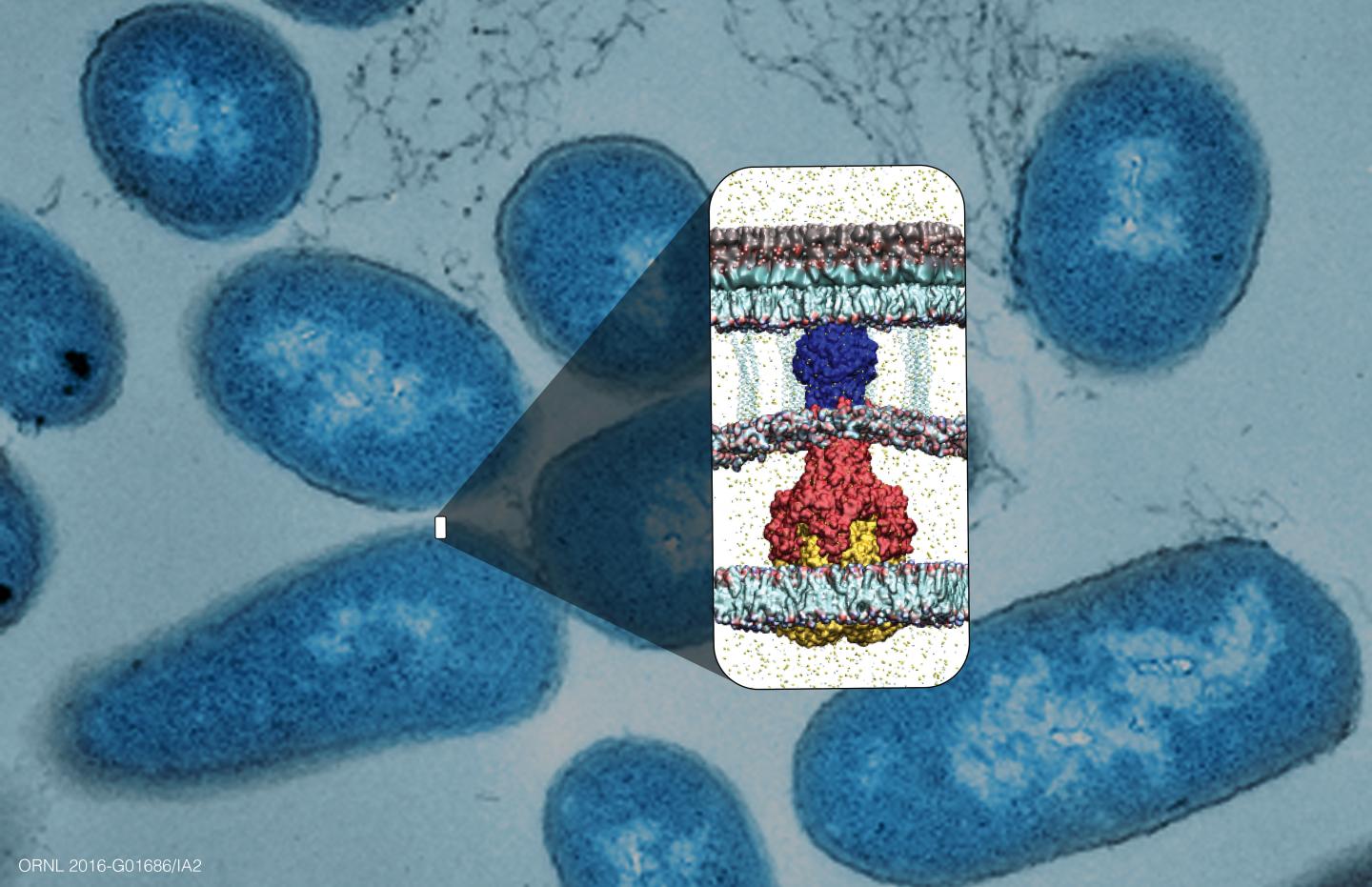
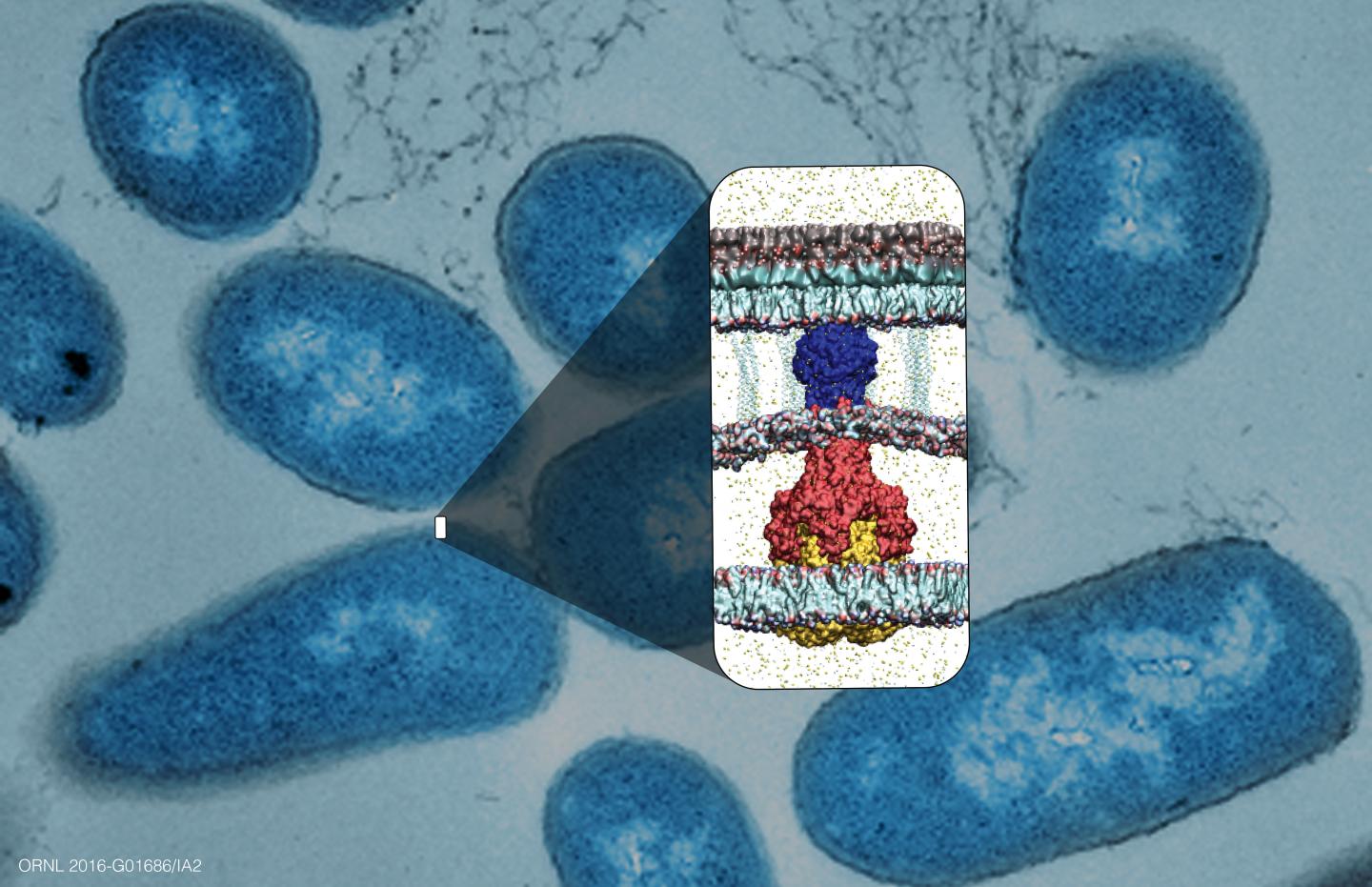
The team focused on one efflux pump protein, known as AcrA, which connects two other proteins in a tunnel shape through the bacterial cell envelope. Disrupting this protein could essentially break the efflux pump–an approach unlike other drug design strategies that try to inhibit the biochemical processes.
"In contrast to previous approaches, our new mechanism uses mechanics to revive an existing antibiotic's ability to fight infection," said Smith, UT-ORNL Governor's Chair and director of the UT-ORNL Center for Molecular Biophysics.
The laboratory experiments were done jointly with extensive protein simulations run on ORNL's Titan supercomputer. Large numbers of chemicals were scanned to predict and select which would be the most effective in preventing AcrA proteins from assembling properly. Using computational models produced by the Titan supercomputer, researchers screened various combinations of molecules and proteins to determine which ones were most disruptive to their formation.
"The first screening took only 20 minutes using 42,000 processors and yielded several promising results," said Parks, ORNL. "After more extensive analysis, we narrowed down our list to predict which molecules were most likely to disrupt the function of the efflux pump."
OU researchers then conducted laboratory experiments to confirm the disruption of the efflux pump and the antibiotic-reviving capability of four of the molecules selected. The SLU School of Medicine research team synthesized structural analogs of the discovered efflux pump inhibitors and identified properties essential for their activities.
###
The study, "Reviving Antibiotics: Efflux Pump Inhibitors That Interact with AcrA, a Membrane Fusion Protein of the AcrAB-ToIC Multidrug Efflux Pump," was published in the American Chemical Society's Infectious Diseases journal. Support for the project was provided by NIH grant number RO1AI052293 in the amount of $2 million. For more information about this project, please contact [email protected].
Media Contact
Jana Smith
[email protected]
405-325-1322
@ouresearch
http://www.ou.edu
############
Story Source: Materials provided by Scienmag