Credit: DNDi
PARIS, 12 April 2018 – An affordable hepatitis C combination treatment including the new drug candidate ravidasvir has been shown to be safe and effective, with extremely high cure rates for patients, including hard-to-treat cases, according to interim results from the Phase II/III STORM-C-1 trial presented by the non-profit research and development organisation Drugs for Neglected Diseases initiative (DNDi) at the International Liver Conference in Paris.
"The results indicate that the sofosbuvir/ravidasvir combination is comparable to the very best hepatitis C therapies available today but it is priced affordably and could allow an alternative option in countries excluded from pharmaceutical company access programs," said Bernard Pécoul Executive Director, DNDi.
The trial using medicines produced by Egyptian drug manufacturer Pharco Pharmaceuticals was run by DNDi and co-sponsored by the Malaysian Ministry of Health, in ten sites in Malaysia and Thailand. Agreements signed in 2016 and 2017 enabling the trials and patient scale-up in Malaysia set out a target price of US$300 for a 12-week treatment, an almost 100% drop from existing treatment prices in Malaysia.
"As hepatitis C has become a major public health concern in Malaysia, it is crucial to increase access to treatment for the benefit of the nation," said Datuk Dr Noor Hisham Abdullah Director General of Health, Ministry of Health, Malaysia. In September 2017, the government of Malaysia issued a "government-use" license on sofosbuvir patents to allow 400,000 people living with hepatitis C in Malaysia to access generic HCV regimens in public hospitals.
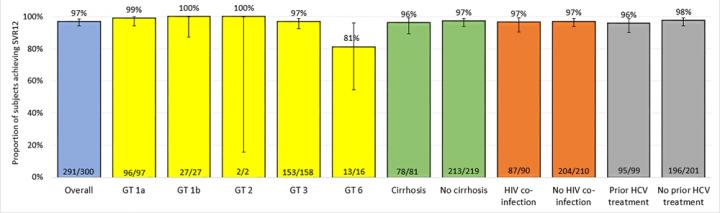
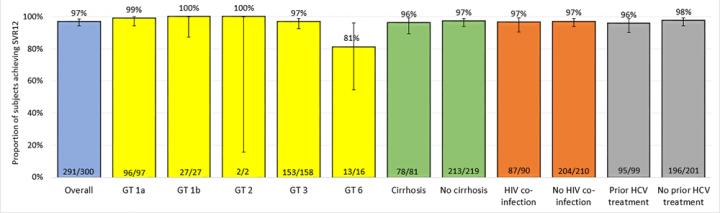
DNDi conducted the STORM-C-1 open label trial to assess the efficacy, safety, tolerance and pharmacokinetics of the drug candidate ravidasvir combined with sofosbuvir. 301 chronically infected adults were treated with the ravidasvir/sofosbuvir combination for 12 weeks for patients without cirrhosis of the liver, and for 24 weeks for those with compensated cirrhosis. In accordance with international standards defining cure for HCV treatments, 12 weeks after treatment completion, 97% of those enrolled were cured (95% CI: 94.4-98.6). Cure rates were very high even for the hardest-to-treat patients: people with liver cirrhosis (96% cured), people living with HIV using their usual treatment (97%), people infected with genotype 3 (97%) including those with cirrhosis (96%), and people who had been exposed to previous HCV treatments (96%). Importantly, patients combining several of these risk factors were cured, and no unexpected safety signals were detected.
"From a treatment provider perspective, this is very exciting as we have been waiting for a simple, affordable, robust treatment tolerated by all patients groups, including those whose treatment outcomes are currently poorer, like patients under antiretroviral therapy," said Pierre Mendiharat, Deputy Operations Director for Médecins Sans Frontières / Doctors Without Borders (MSF). "This will be crucial to expand treatment to the most vulnerable categories of patients in developing countries."MSF and DNDi are working together to increase access to care and treatment for HCV patients in key low- and middle-income countries, through the STORM-C project financed by MSF's Transformational Investment Capacity (TIC) initiative.
Over 71 million people live with hepatitis C worldwide, a disease which causes 400,000 deaths a year. Although highly effective treatments have existed for a number of years, less than three million people are on treatment, with more people infected every year than are put on treatment. The World Health Organization aims for 80% of people diagnosed with HCV to be put on treatment by 2030.
Ravidasvir is an oral NS5A inhibitor licensed to DNDi by Presidio Pharmaceuticals. Most people enrolled in the DNDi trial in Malaysia and Thailand had genotype 1 (42% of participants) or genotype 3 (53%), thereby confirming the combination's effectiveness for those two additional genotypes. Further trials are planned to document the efficacy and safety of the combination in patients infected with the other HCV genotypes and in particularly vulnerable groups, to enable a public health approach to the treatment of hepatitis C.
"Pharco is proud to enable a public health approach to hepatitis C treatment by providing affordable treatments. We look forward to future collaboration in additional clinical trials to confirm the safety and efficacy of ravidasvir," said Dr. Sherine Helmy, CEO, Pharco.
###
Media Contacts
DNDi: [email protected] or Ilan Moss +1 646 266 5216, [email protected]
Poster reference: Isabelle Andrieux-Meyer, Tan Soek Siam,Nicolas Salvadori, François Simon, Tim R. Cressey, Hajiah Rosaida Hi Mohd Said, Muhammad Radzi Abu Hassan, Haniza Omar, Hoi-Poh Tee, Chan Wah Kheong, Goh Khean Lee, Sharifah Faridah Syed Omar, Adeeba Kamarulzaman, Suresh Kumar, Satawat Thongsawat, Kanawee Thetket, Anchalee Avihingsanon, Suparat Khemnark, Sombat Thanprasertsuk, Jean-Michel Piedagnel, Sasikala Siva, Nur Asimah, Nelson Da Silva, Jennifer Brenner, Bernard Pecoul, Marc Lallemant, Shahnaz Murad. Safety and efficacy of ravidasvir plus sofosbuvir 12 weeks in non-cirrhotic and 24 weeks in cirrhotic patients with hepatitis C virus genotypes 1, 2, 3 and 6: the STORM-C-1 phase II/III trial. International Liver Congress, Paris, April 11-15 2018, France. Poster LBP-032.
About DNDi
The Drugs for Neglected Diseases initiative (DNDi) is a not-for-profit R&D organization working to deliver new treatments for neglected patients, in particular sleeping sickness, Chagas disease, leishmaniasis, filaria, paediatric HIV/AIDS, and hepatitis C. http://www.dndi.org
Media Contact
Ilan Moss
[email protected]
646-266-5216
@DNDi
Original Source
https://www.dndi.org/2018/media-centre/press-releases/new-affordable-hepatitis-c-combination-treatment-shows-97-cure-rate