Credit: ORNL
Neutron analysis at the Department of Energy's Oak Ridge National Laboratory is helping researchers better understand a key enzyme found in a bacterium known to cause stomach cancer.
Understanding the details of this enzyme, and thus the Helicobacter pylori bacteria's metabolism and biological pathways, could be central to developing drugs that act against H. pylori, but that do not attack the stomach's useful bacteria.
"Most drugs, including common antibiotics, use a generalized mechanism to bind to their targets, which, in turn, eliminates the good bacteria you need to stay healthy, as well as the bad bacteria," said Andrey Kovalevsky, one of the instrument scientists at the ORNL High Flux Isotope Reactor and coauthor of this research published in the Proceedings of the National Academy of Sciences.
"By understanding how this enzyme functions, we can get clues about how to fine-tune a drug to recognize only a specific target, which would eliminate some of the side effects that cause so many problems for people when a more generalized approach to kill bacteria is used."
Kovalevsky was part of a team led by Donald Ronning at the University of Toledo who used HFIR's IMAGINE instrument to study the metabolism of a bacterial enzyme known as H. pylori 5'-methylthioadenosine nucleosidase, or HpMTAN, which plays a key function in H. pylori. This bacterium garnered international attention in 2005 when a team of researchers was awarded the Nobel Prize in Medicine for determining its role as a "bacterial culprit" in the development of gastric conditions, including ulcers, chronic gastritis and cancer.
Ronning's team focused on H. pylori's use of a unique biosynthetic pathway to synthesize vitamin K2, which aids in the electron transfer processes, or chemical reactions, of all organisms. HpMTAN is one specific enzyme that functionbs within this unique pathway and provides the promising specific target or point of attack for new medications. Vitamin K2 acts to expedite the HpMTAN enzyme's interaction with other macromolecules, including the very bacterium that causes an array of gastric health issues.
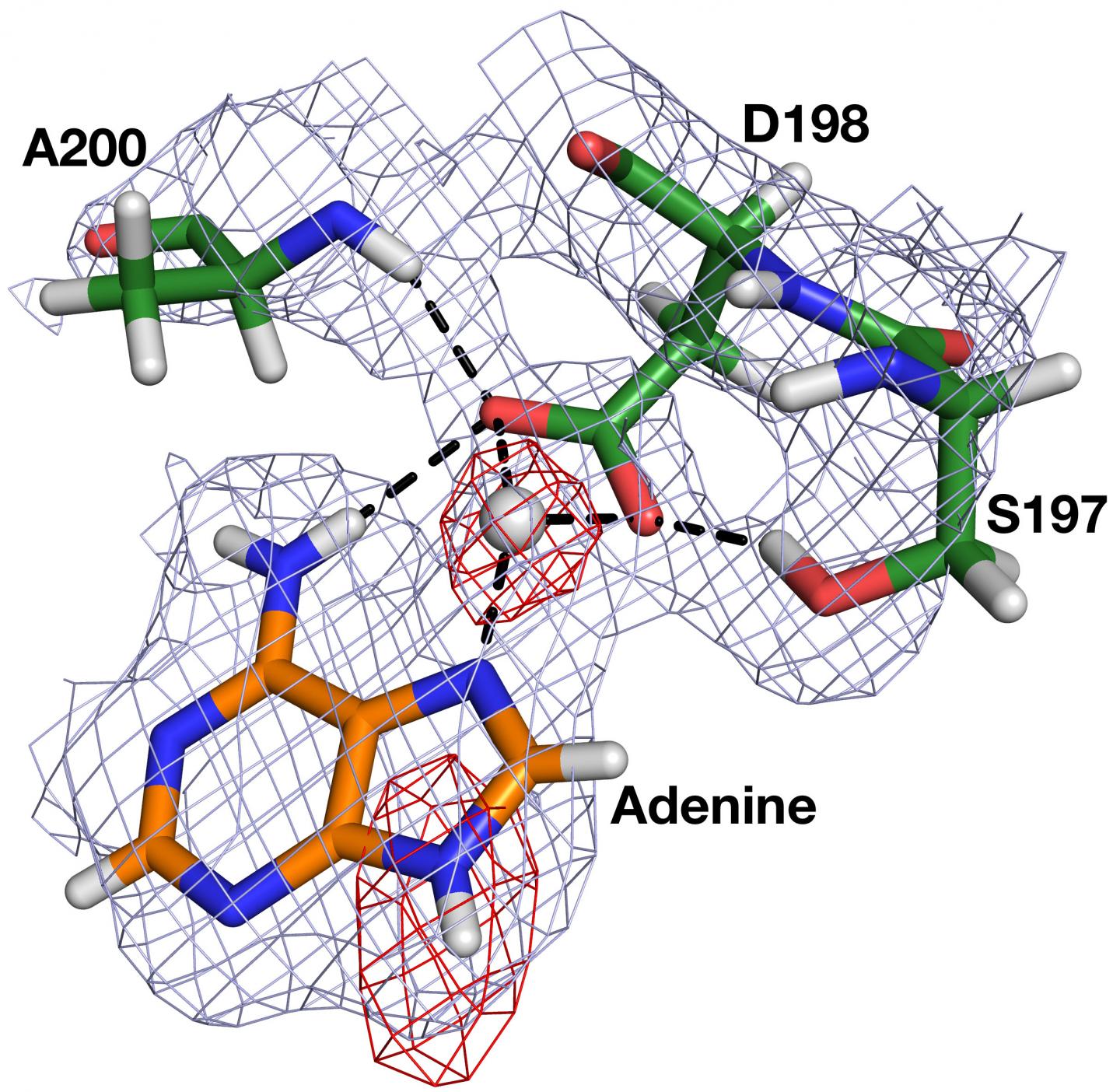
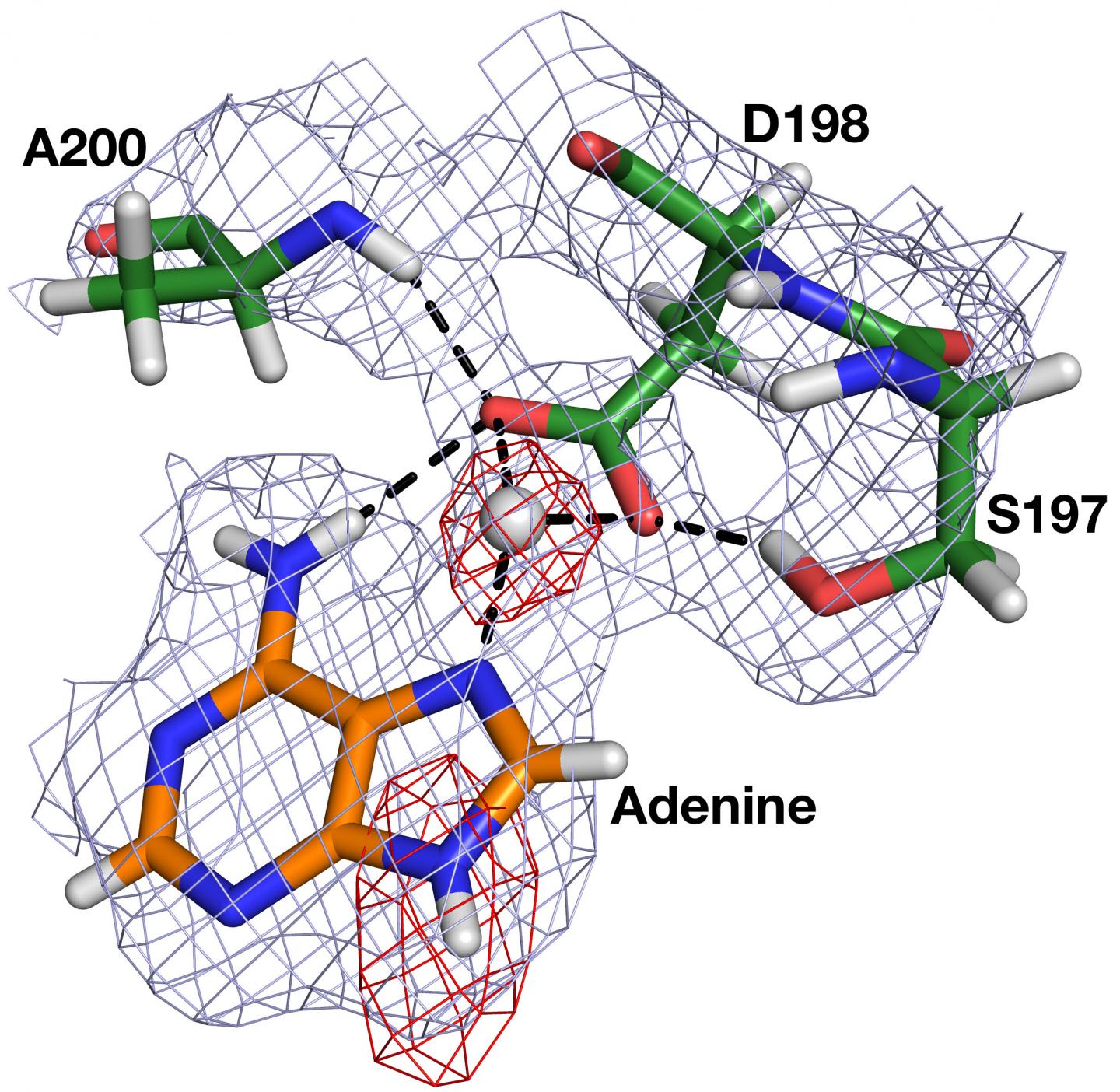
Neutron crystallography at HFIR's IMAGINE instrument allowed researchers to accurately visualize the positions and predict the movements of hydrogen atoms in HpMTAN, especially those involved in the critical stages when the enzyme binds to its substrate and then proceeds with the catalytic reaction.
For a comprehensive view on the interatomic interactions, Ronning's team examined four different HpMTAN neutron structures to observe how ligands, or molecules that bind via noncovalent bonding, interacted with their respective enzyme sites.
"This knowledge will inform future drug design efforts by taking advantage of the known orientation of the nucleophilic water molecule and its intimate interactions with the neighboring components of the enzyme," Ronning said.
While Ronning qualified that the development of a new drug to treat gastric issues will take several years and continued study of the enzyme's behavior, he said that his team's research confirms HpMTAN's potential for use and this knowledge could, in fact, speed up the creation of such a medicine.
###
This research was partially funded through the National Institute of Health's National Institute of Allergy and Infectious Disease and a cooperative agreement with the National Aeronautics and Space Administration's Center for the Advancement of Science in Space. The work was conducted in part at the Heinz Maier-Leibnitz Research Neutron Source with the Technical University of Munich, both in Munich, Germany, and at ORNL's High Flux Isotope Reactor, a DOE Office of Science User Facility.
UT-Battelle manages ORNL for the DOE's Office of Science. The Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit http://science.energy.gov/.
Media Contact
Katie Bethea
[email protected]
865-576-8039
@ORNL
http://www.ornl.gov