The low-cost, efficient production of hydrogen is an important step toward developing alternative, clean energy sources. Electrochemical water splitting, which splits water into its hydrogen and oxygen elements using an electrocatalyst, is a viable option for producing hydrogen. Conventionally, catalysts have been based on costly elements such as platinum, which makes it difficult to apply this technology on a widespread, commercial scale.
Credit: Nano Research, Tsinghua University Press
The low-cost, efficient production of hydrogen is an important step toward developing alternative, clean energy sources. Electrochemical water splitting, which splits water into its hydrogen and oxygen elements using an electrocatalyst, is a viable option for producing hydrogen. Conventionally, catalysts have been based on costly elements such as platinum, which makes it difficult to apply this technology on a widespread, commercial scale.
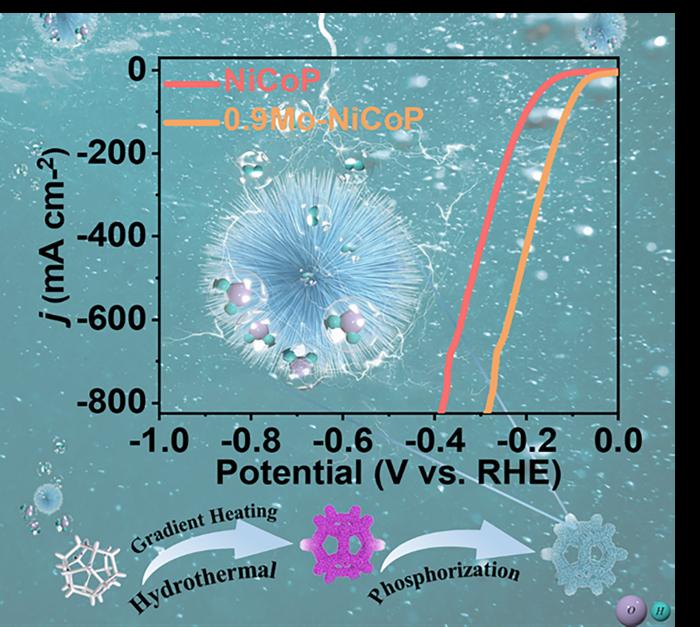
In a recently published paper, researchers demonstrated how adding molybdenum to a nickel-cobalt phosphide catalyst and synthesizing it with a gradient hydrothermal process, in which the catalyst is heated to 100 degrees, 150 degrees, and then 180 degrees Celsius over 10 hours, created a unique microstructure that improved the performance of the catalyst, resulting in hydrogen production that could be more applicable to large-scale hydrogen production.
The paper was published in Nano Research on July 26.
“The innovative combination of gradient hydrothermal and phosphidation processes forms a microsphere structure,” said Yufeng Zhao, a professor at the College of Sciences & Institute for Sustainable Energy at Shanghai University in Shanghai, China. “These nanoparticles with a diameter of approximately 5 to 10 nanometers form nanoneedles, which subsequently self-assemble into a spherical structure. The nanoneedles offer abundant active sites for efficient electron transfer and the presence of small-sized particles and micro-scale roughness enhances the release of hydrogen bubbles.”
To create this unique microstructure, researchers employed a technique called element doping. Element doping is the intentional addition of impurities to a catalyst to improve its activity. In this study, molybdenum (Mo) was added to the bimetallic nickel-cobalt (Ni-Co) phosphide (P). Ni-Co phosphides already have exceptional electrocatalytic performance because of the way the cobalt and nickel ions interact. After adding the molybdenum and then using a gradient hydrothermal process, the Mo-doped Ni-CoP was deposited onto a nickel foam. After this process, the unique microstructure of nanoneedles formed on the phosphide.
“Trace molybdenum doping optimizes the electronic structure and increases the number of electroactive sites,” said Zhao. The Mo-doped Ni-CoP catalyst was tested for reliability, stability, and performance. Its density remained nearly constant after 100 hours and its structure was well-maintained, thanks in part to the unique structure of the nanoneedles, which prevent the catalyst from collapsing as hydrogen accumulates. Calculations also showed that the phosphide catalyst was exceptionally efficient.
Looking ahead, researchers hope to test the performance of the reaction in different solutions, such as acidic and neutral solutions. Future studies will also look at alternatives to nickel foam, such as titanium mesh, that can operate across the pH range. “In future work, we recommend exploring the application of the catalyst in the oxidation-assisted hydrogen production of small molecules, such as urea. This approach would reduce the overpotential of water electrolysis and mitigate environmental pollution caused by urea wastewater,” said Zhao.
Other contributors include Chengyu Huang, Zhonghong Xia, Jing Zhang, Chenfei Zhao, Jiujun Zhang, and Shengjuan Huo of the College of Sciences & Institute for Sustainable Energy Shanghai University; Jing Wang at the State Key Laboratory of Metastable Materials at Yanshan University; Xingli Zou and Xionggang Lu at the State Key Laboratory of Advanced Special Steel & Shanghai Key Laboratory of Advanced Ferrometallurgy & School of Materials Science and Engineering at Shanghai University; Shichun Mu at the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing at Wuhan University of Technology; and HongJin Fan at the School of Physical and Mathematical Sciences at Nanyang Technological University.
The National Natural Science Foundation of China (22179077), the National Natural Science Foundation Youth Fund (22209104), Shanghai Science and Technology Commission’s “2020 Science and Technology Innovation Action Plan” (20511104003), the Natural Science Foundation of Shanghai (21ZR1424200), Hebei Provincial Department of Science and Technology (226Z4404G), and the Hebei Science Foundation (E2021203005) supported this research.
##
About Nano Research
Nano Research is a peer-reviewed, international and interdisciplinary research journal, publishes all aspects of nano science and technology, featured in rapid review and fast publishing, sponsored by Tsinghua University and the Chinese Chemical Society. It offers readers an attractive mix of authoritative and comprehensive reviews and original cutting-edge research papers. After 15 years of development, it has become one of the most influential academic journals in the nano field. In 2023 InCites Journal Citation Reports, Nano Research has an Impact Factor of 9.9, the total cites reached 35645, ranking first in China’s international academic journals, and the number of highly cited papers reached 194, ranked among the top 1.8% of 8769 academic journals.
About Tsinghua University Press
Established in 1980, belonging to Tsinghua University, Tsinghua University Press (TUP) is a leading comprehensive higher education and professional publisher in China. Committed to building a top-level global cultural brand, after 42 years of development, TUP has established an outstanding managerial system and enterprise structure, and delivered multimedia and multi-dimensional publications covering books, audio, video, electronic products, journals and digital publications. In addition, TUP actively carries out its strategic transformation from educational publishing to content development and service for teaching & learning and was named First-class National Publisher for achieving remarkable results.
Journal
Nano Research
DOI
10.1007/s12274-023-5892-7
Article Title
Highly Efficient and Stable Electrocatalyst for Hydrogen Evolution by Molybdenum Doped Ni-Co Phosphide Nanoneedles at High Current Density
Article Publication Date
26-Jul-2023




