In a chemical reaction, molecules in different substances meet one another to form new molecules causing changes in the bonds of their atoms. The molecules collide at an extremely close distance—a nanometer or less—in an extremely short amount of time. This makes investigating the details of chemical reactions at the molecular scale difficult. Most experimental knowledge, used to explain single-molecule reaction dynamics, was obtained by studying reactions in gases. However, the overwhelming majority of chemical reactions take place in liquids, so elucidating single-molecule reaction dynamics in solution is an important challenge, with very few experimental tools.
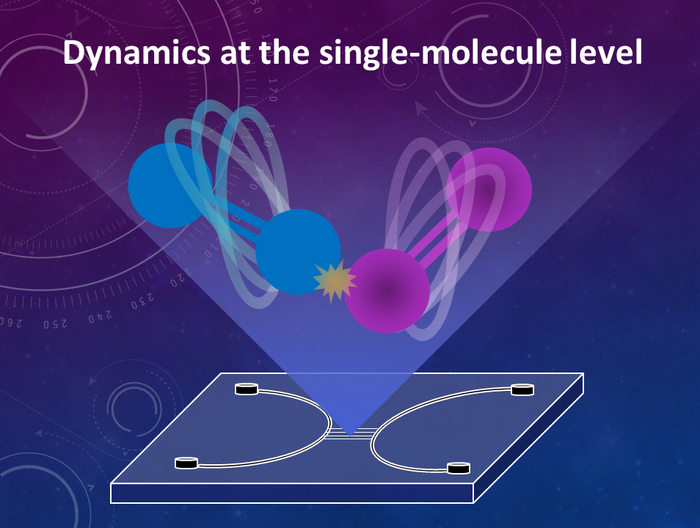
Credit: Yan Xu, Osaka Metropolitan University
In a chemical reaction, molecules in different substances meet one another to form new molecules causing changes in the bonds of their atoms. The molecules collide at an extremely close distance—a nanometer or less—in an extremely short amount of time. This makes investigating the details of chemical reactions at the molecular scale difficult. Most experimental knowledge, used to explain single-molecule reaction dynamics, was obtained by studying reactions in gases. However, the overwhelming majority of chemical reactions take place in liquids, so elucidating single-molecule reaction dynamics in solution is an important challenge, with very few experimental tools.
A nanofluidic device—a few square centimeters of glass plate with nanometer-sized nanofluidic channels carved into it—provides a test tube-like environment to confine individual molecules. But nanofluidic devices have the potential to be used in combination with various existing analytical instruments with high temporal resolution, to investigate extremely fast single molecule reactions.
The authors of the review, Associate Professor Yan Xu and Dr. Nattapong Chantipmanee of the Osaka Metropolitan University Graduate School of Engineering, have engineered principles and technologies to freely manipulate nanomaterials, biomaterials, and molecules at the single-molecule level. The methodologies covered by their review use fundamental technologies such as nanofluidic processing, functional integration, and fluidic control and measurement, pioneering the way to integrate various fields by using nanofluidics. In addition, to elucidate the single molecule dynamics of reactions in solution using their unique nanofluidic devices, they are working to solve problems such as how to precisely manipulate small molecules in solution and how to measure their extremely quick—nano- to picosecond—reactions.
The researchers published their review article on single-molecule reaction dynamics in solution pioneered by nanofluidic devices, in the January 2023 issue of TrAC Trends in Analytical Chemistry. As pioneers in this new field, the review provides a bird’s eye view, including the forefront of research and development, future challenges, and where new these discoveries may lead.
“Nanofluidic devices have the potential to become a fantastic experimental tool to elucidate the dynamics of solution reactions. I hope this paper will encourage more researchers to join this budding field of research,” said Professor Xu.
###
About OMU
Osaka Metropolitan University is a new public university established in April 2022, formed by merger between Osaka City University and Osaka Prefecture University. For more research news visit https://www.omu.ac.jp/en/ or follow @OsakaMetUniv_en and #OMUScience.
Journal
TrAC Trends in Analytical Chemistry
DOI
10.1016/j.trac.2022.116877
Method of Research
Literature review
Subject of Research
Not applicable
Article Title
Nanofluidics for chemical and biological dynamics in solution at the single molecular level
Article Publication Date
7-Dec-2022