Two proteins that act as a ‘clutch’ in cells to put them in gear and drive our immune response have been identified for the first time
Credit: Dr. Darius Köster, University of Warwick
- International team including University of Warwick scientists identifies proteins that drive activation of our immune response
- Adaptor proteins act as a ‘clutch’ to move clusters of proteins within cells
- Could open opportunities to design immune cells to combat specific problems
- Protein condensates are involved; have been found to play roles in many biological processes and diseases, including Huntington’s disease, amyotrophic lateral sclerosis, and types of cancer
Two proteins that act as a ‘clutch’ in cells to put them in gear and drive our immune response have been identified for the first time.
A team of biochemists and cell biologists–gathered from the University of Warwick (UK), the University of Texas Southwestern (UTSW) Medical Center (USA), University of California, San Francisco (UCSF) (USA), and from the National Centre for Biological Sciences (NCBS-TIFR), Bangalore (India) — working together at the Marine Biological Laboratory, Woods Hole in the United States thanks to funding by the Howard Hughes Medical Institute — have uncovered a process within cells that shows how they move contents around inside them. It appears that they move in a manner similar to switching gears in a car.
The research, published in the journal eLife, could give insights into the mechanisms that activate immune cells and could eventually drive the development of new treatments.
The research focused on the composition of protein condensates – clusters of different types of proteins bound together that are found inside cells. These condensates have been found to play significant roles in many biological processes, and have also been implicated in diseases, including Huntington’s disease, amyotrophic lateral sclerosis, and several types of cancer.
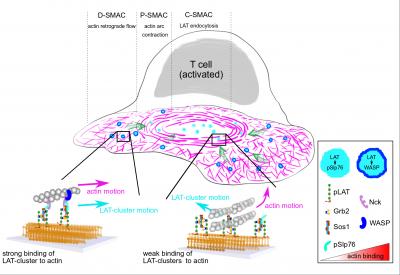
One system that protein condensates play an important role in is the activation of T cells, which are very important for producing antibodies and for communicating to the rest of the body that there is an infection present. T cells are constantly screening for small amounts of antigen presenting cells, which are vital for an effective adaptive immune response, so have to be easily but accurately triggered.
When a T cell binds to an antigen presenting cell, the T cell receptors are activated, and a cascade of processes are triggered. The T cell starts to rearrange its cortex and create a zone around these receptors called the immunological synapse.
A network of filaments within the cell made from actin guides a condensate carrying a protein called LAT from the cell periphery towards the centre of the cortex continuously to keep the T cell activated.
The researchers were able to demonstrate that two adaptor proteins, Nck and N-WASP/WASP, act like a ‘clutch’ in a car, allowing the condensate to slot into the correct gear position and speed up its progress to the centre of the cell.
The discovery sheds light on the control mechanisms for the activation of our immune response, and potentially could open opportunities to design T cells that are only active for particular problems.
Dr. Darius Köster, an Assistant Professor at the Centre for Mechanochemical Cell Biology – Warwick Medical School, explains: “Proteins condensates have distinct compositions and distinct preferential locations within cells, and they are associated with distinct biological functions, including DNA replication, RNA metabolism, signal transduction, synaptic transmission, and stress response.
“For this research, colleagues rebuilt these condensates in vitro to demonstrate that LAT can be the seed for forming these protein assemblies. We then combined this system with a rebuilt actin cortex system to get a better understanding of what happens to phase-separating protein bunches in the vicinity of an actively moving actin network.
“Depending on which modular molecules are used in the LAT clusters, their interaction with actin changes. It’s a bit like a clutch in your car, some molecules interact weakly with the actin, but by adding another molecule they will interact much more strongly.
“Using this reconstituted system allowed us to make much more minute changes to the protein condensate composition that would not be so easy to do in the live cell.”
Professor Satyajit Mayor (NCBS-TIFR) commented on the unique way in which scientists from different institutes and continents came together with their respective experience and expertise to collaborate on solving a central question that is emerging in the new area of phase separating membrane-less molecular assemblies.
He comments: “This effort was made possible by a unique collaboration. An idea supported by Howard Hughes Medical Institute (HHMI), produced the ‘HHMI/MBL Summer Institute’, (organized primarily by the HHMI investigators Mike Rosen (UTSW), Ron Vale (UCSF) and Jim Wilhelm (UC San Diego) to study the mechanisms that control the composition and consequent function of these exciting phases in living cells. While the lead author of this study, Jon, was reconstituting the phase separating T Cell receptor signalling complex (at UTSW) along with Xiaolei Su (at UCSF), Darius brought his in vitro actomyosin membrane cortex (developed at the NCBS) to the heady collaborative atmosphere of the Marine Biological Laboratory in Woods Hole, Massachusetts. The natural consequence was to mix one system with the other. The understanding gained from this active mixture provided crucial insights into the functioning of the molecular clutch that couples the T cell signalling complex with a centripetally moving acting cytoskeleton. This coupling in turn regulates the function of T cell receptors in aiding the immune system to recognize foreign antigens.”
Dr. Michael Rosen echoes this sentiment, adding: “The Summer Institute brought together scientists from across the globe to participate in a unique collaborative environment at the MBL. By living and working together for eight weeks over several summers, we were able to make scientific discoveries that would have been impossible for any of our groups individually. The work described in our eLife paper, combining extremely complex biochemistry with cutting-edge imaging and image analysis, exemplifies the spirit and accomplishments of the Summer Institute.”
###
- ‘A composition-dependent molecular clutch between T cell signaling condensates and actin’ published in eLife, DOI: 10.7554/eLife.42695
- More information about the work at the HHMI/MBL Summer Institute: https:/
/ www. utsouthwestern. edu/ research/ in-pursuit/ archive/ year-2018/ woods-hole. html - Rosen lab: https:/
/ www. utsouthwestern. edu/ labs/ rosen/ - Mayor lab: https:/
/ www. ncbs. res. in/ faculty/ mayor - Köster lab: http://mechanochemistry.
org/ Koester
Media Contact
Peter Thorley
[email protected]
Related Journal Article
http://dx.




