Each year, an estimated 101,000 children below the age of 5 years die across the globe due to infections caused by respiratory syncytial virus (RSV). Worldwide, RSV is a main cause of hospitalisation among young children. [1]
It took more than 60 years to develop and approve vaccines against RSV that can now be used to immunise both elderly people and pregnant women in the European Union (EU). In addition, a long-acting monoclonal antibody (nirsevimab) was licensed in the EU for use in infants end of 2022. Both events marked a milestone in the attempt to prevent RSV infections especially in very young children.
In an editorial in Eurosurveillance, Eeva Broberg and Hanna Nohynek [2] look at these recent advances and which supporting measures might be needed to understand the actual burden of RSV, its annual circulation patterns and genomic evolution.
For that understanding to improve, Broberg and Nohynek argue that “sequencing data need to be collected and reported to publicly accessible databases. As for other respiratory viruses and most infectious diseases, under-ascertainment will remain an issue for RSV surveillance in general as patients may present late for testing after the infection or not even be tested if they present with mild symptoms.”
Overcoming the challenges of sequencing RSV
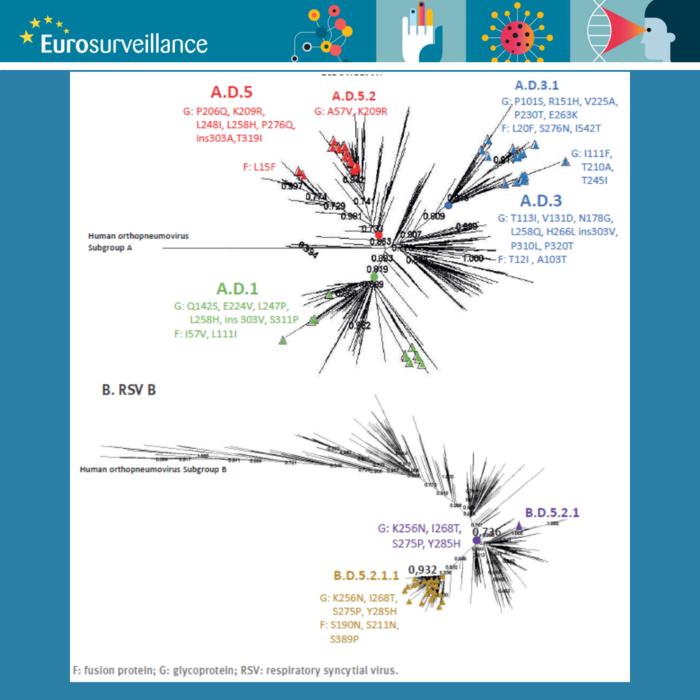
To illustrate the current changes in tackling RSV infections across Europe, Broberg and Nohynek relate to the rapid communication in the same Eurosurveillance issue. Iglesias-Caballero et al. describe two PCR-based sequencing systems for RSV that can be used as methods to monitor the genetic diversity of the virus and with that inform molecular epidemiology, vaccine effectiveness and treatment strategies.
The authors show that their approach is less labour-intensive and less costly and presents the opportunity to increase laboratory capacities and probably also shorten throughput times. According to Broberg and Nohynek, the study from Spain demonstrates that the method outlined by Iglesias-Caballero et al. “can be used in retrospective molecular epidemiological studies of RSV and even with the simplified approach to sequence only the G and F gene genome, molecular evolution of RSV could be assessed.”
Complementing this RSV article from Spain, Martinón-Torres et al. [3] review first experiences in the autonomous community Galicia (northern Spain) following the implementation of universal RSV prophylaxis in infants with long-acting monoclonal antibodies. Both Spain and France have already introduced nirsevimab in their national immunisation programmes.
Based on the findings in Galicia, the early uptake of nirsevimab, which had a range of 81–98% in this region according to the study, has exceeded expectations. This immunisation achievement is the result of successful targeting and rationally designed immunisation logistics. According to the authors, the implementation and motivation of parents to immunise their children was likely facilitated by the quite intense RSV epidemic in Europe during the autumn 2022 in combination with awareness raising campaigns.
As Broberg and Nohynek put it, “the value of the work of Martinón-Torres et al. lies not only in the clear and meticulous description of how implementation challenges were approached, i.e. by distinguishing infants belonging to the seasonal and catch-up or high-risk groups, which provides an excellent example to other European countries, but also in the fact that Galicia will be able to answer the burning question on whether universal introduction of nirsevimab in the real- world setting is a cost-effective intervention on the short as well as the long run. If preventing RSV infection in early life can reduce asthma later in life, the impact of the intervention will be manifold.”
The European Centre for Disease Prevention and Control and the World Health Organization Regional Office for Europe have agreed on an integrated respiratory surveillance, which also includes RSV, and the weekly results are published in the European Respiratory Virus Surveillance Summary
Credit: Eurosurveillance
Each year, an estimated 101,000 children below the age of 5 years die across the globe due to infections caused by respiratory syncytial virus (RSV). Worldwide, RSV is a main cause of hospitalisation among young children. [1]
It took more than 60 years to develop and approve vaccines against RSV that can now be used to immunise both elderly people and pregnant women in the European Union (EU). In addition, a long-acting monoclonal antibody (nirsevimab) was licensed in the EU for use in infants end of 2022. Both events marked a milestone in the attempt to prevent RSV infections especially in very young children.
In an editorial in Eurosurveillance, Eeva Broberg and Hanna Nohynek [2] look at these recent advances and which supporting measures might be needed to understand the actual burden of RSV, its annual circulation patterns and genomic evolution.
For that understanding to improve, Broberg and Nohynek argue that “sequencing data need to be collected and reported to publicly accessible databases. As for other respiratory viruses and most infectious diseases, under-ascertainment will remain an issue for RSV surveillance in general as patients may present late for testing after the infection or not even be tested if they present with mild symptoms.”
Overcoming the challenges of sequencing RSV
To illustrate the current changes in tackling RSV infections across Europe, Broberg and Nohynek relate to the rapid communication in the same Eurosurveillance issue. Iglesias-Caballero et al. describe two PCR-based sequencing systems for RSV that can be used as methods to monitor the genetic diversity of the virus and with that inform molecular epidemiology, vaccine effectiveness and treatment strategies.
The authors show that their approach is less labour-intensive and less costly and presents the opportunity to increase laboratory capacities and probably also shorten throughput times. According to Broberg and Nohynek, the study from Spain demonstrates that the method outlined by Iglesias-Caballero et al. “can be used in retrospective molecular epidemiological studies of RSV and even with the simplified approach to sequence only the G and F gene genome, molecular evolution of RSV could be assessed.”
Complementing this RSV article from Spain, Martinón-Torres et al. [3] review first experiences in the autonomous community Galicia (northern Spain) following the implementation of universal RSV prophylaxis in infants with long-acting monoclonal antibodies. Both Spain and France have already introduced nirsevimab in their national immunisation programmes.
Based on the findings in Galicia, the early uptake of nirsevimab, which had a range of 81–98% in this region according to the study, has exceeded expectations. This immunisation achievement is the result of successful targeting and rationally designed immunisation logistics. According to the authors, the implementation and motivation of parents to immunise their children was likely facilitated by the quite intense RSV epidemic in Europe during the autumn 2022 in combination with awareness raising campaigns.
As Broberg and Nohynek put it, “the value of the work of Martinón-Torres et al. lies not only in the clear and meticulous description of how implementation challenges were approached, i.e. by distinguishing infants belonging to the seasonal and catch-up or high-risk groups, which provides an excellent example to other European countries, but also in the fact that Galicia will be able to answer the burning question on whether universal introduction of nirsevimab in the real- world setting is a cost-effective intervention on the short as well as the long run. If preventing RSV infection in early life can reduce asthma later in life, the impact of the intervention will be manifold.”
The European Centre for Disease Prevention and Control and the World Health Organization Regional Office for Europe have agreed on an integrated respiratory surveillance, which also includes RSV, and the weekly results are published in the European Respiratory Virus Surveillance Summary
—-Ends—-
References/notes to editors:
[1] Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047-64. https://doi.org/10.1016/S0140-6736(22)00478-0 PMID: 35598608
[2] Broberg Eeva K, Nohynek Hanna. Respiratory syncytial virus infections – recent developments providing promising new tools for disease prevention. Euro Surveill. 2023;28(49):pii=2300686. https://doi.org/10.2807/1560-7917.ES.2023.28.49.2300686
[3] Martinón-Torres Federico, Mirás-Carballal Susana, Durán-Parrondo Carmen. Early lessons from the implementation of universal respiratory syncytial virus prophylaxis in infants with long-acting monoclonal antibodies, Galicia, Spain, September and October 2023. Euro Surveill. 2023;28(49):pii=2300606. https://doi.org/10.2807/1560-7917.ES.2023.28.49.2300606
[4] Respiratory syncytial virus (RSV) is a common respiratory virus that causes mild, cold-like symptoms. People who contract RSV usually recover in around a week without the need for medical treatment. However, in infants under six months of age, people over 65, and people with a compromised immune system, RSV can cause severe illness and death. Symptoms usually appear two to eight days after being infected. Read more: https://www.ecdc.europa.eu/en/respiratory-syncytial-virus-rsv
Journal
Eurosurveillance
DOI
10.2807/1560-7917.ES.2023.28.49.2300606
Method of Research
Commentary/editorial
Article Title
Respiratory syncytial virus infections – recent developments providing promising new tools for disease prevention
Article Publication Date
7-Dec-2023
COI Statement
Hanna Nohynek: Co-lead of Work package 2 of the Innovate Health Initiative’s Preparing for RSV Immunisation and Surveillance in Europe (PROMISE) project (https://imi-promise.eu), member of the National Immunization Technical Advisory Group of the Finnish Institute for Health and Welfare, and chair of the Strategic Advisory Group of Experts of the World Health Organization.




