Credit: Bruhn, J. et al. Front. Mol. Biosci., 2021 8, 354.
CAMBRIDGE July 13, 2021 – To date, solving structures of potential therapeutics using X-ray diffraction (XRD) has been an assumed, pivotal step in the drug development process. But a recent paper by a team of researchers led by NanoImaging Services shows how microcrystal electron diffraction (MicroED) is growing to obtain the structures of potential pharmaceuticals.
Three-dimensional crystal structures that show the relative positions of atoms, bonds and intramolecular interactions are needed to understand stability, reactivity, solubility and, ultimately, suitability for pharmaceutical use. Pharmaceutical researchers usually use X-ray diffraction (XRD) – with single-crystal XRD preferred – to solve crystal structures. But XRD usually requires large (100 μm or larger), well-ordered crystals and several thousand known active pharmaceutical ingredients (APIs) are available only as crystalline powders that do not readily form large crystals.
“Growing large crystals is a huge bottleneck for those interested in determining crystal structures,” said author Dr. Jessica Bruhn, Scientific Group Leader – MicroED at NanoImaging Services. “MicroED can work with crystals of almost any size as it is generally fairly straightforward to break large crystals into a size suitable for MicroED.”
Developments in automated data collection and data processing have led to increased interest in electron diffraction as an XRD alternative. Electron diffraction is like XRD, except that it uses a beam of electrons rather than X-rays to obtain structures. Since electrons readily interact with matter, MicroED can solve high-resolution crystal structures from sub-micron-sized crystals. It is especially exciting for small-molecule drugs, many of which readily form microcrystals, and the approach helps with the drug discovery phase when sample quantities are extremely limited. In the development phase, researchers can use it to determine structures of reaction products and by-products, which can help guide synthesis strategies and inform production decisions.
“Single-crystal X-ray diffraction is faster, cheaper and easier to access compared to electron diffraction today,” said Bruhn. “However, I do expect to see electron diffraction determining more and more structures inaccessible to X-rays, such as those of transient polymorphs, helping to expand the breadth of crystal structures that can be determined.”
Polymorphs are crystalline structures with the same chemical composition but different molecular packings with different lattice properties, like diamond and graphite. Most active pharmaceutical ingredients (APIs) are thought to exist in more than one polymorphic form, which can give rise to drastically different drug properties. Successful drug formulation requires selecting the optimal polymorph, and that requires easily solving structures. In addition, most drug substances being developed today exhibit poor solubility. Determining the structures of the many different forms an API can adopt (including polymorphs, co-crystals, hydrates, solvates, etc.) helps researchers better engineer optimal crystal forms with good pharmacokinetic properties.
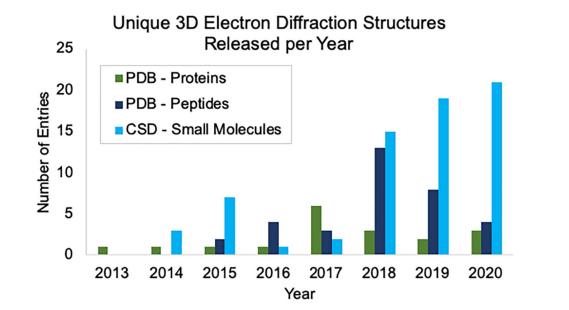
In developing their MicroED pipeline, the researchers explored the prevalence of MicroED data available, including data stored in the Cambridge Structural Database (CSD). The CSD houses small-molecule organic and metal-organic experimental crystal structures with entries enriched and annotated by experts. About 98% of the structures in the CSD are from laboratory X-ray diffractometers, but the CSD houses a growing number of 3D electron diffraction datasets, including those solved by MicroED. Currently, there are over 100 unique datasets determined using electron diffraction in the CSD’s June 2021 web and desktop offerings. In the past three years, the number of electron structures in the CSD has been increasing more rapidly, and the Cambridge Crystallographic Data Centre (CCDC) is committed to supporting scientists worldwide who are depositing and sharing their MicroED data globally.
Suzanna Ward is the Head of Database at the CCDC.
“Electron diffraction is truly one of the most exciting and rapidly evolving areas of structural science,” Ward said. “Recent publications already show how it could help to speed up the development of new drugs, and we are eagerly anticipating how it might impact the volume and breadth of data we are able to share through the CSD. I think we have an interesting journey ahead of us, and it will be intriguing to see how 3D electron diffraction will be utilized in both industry and academia in the coming years.”
###
Journal Citation
Bruhn, J. et al. Front. Mol. Biosci., 2021 8, 354.
Media Contact
Ashley Moreno
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




