Credit: Kanazawa University
[Background]
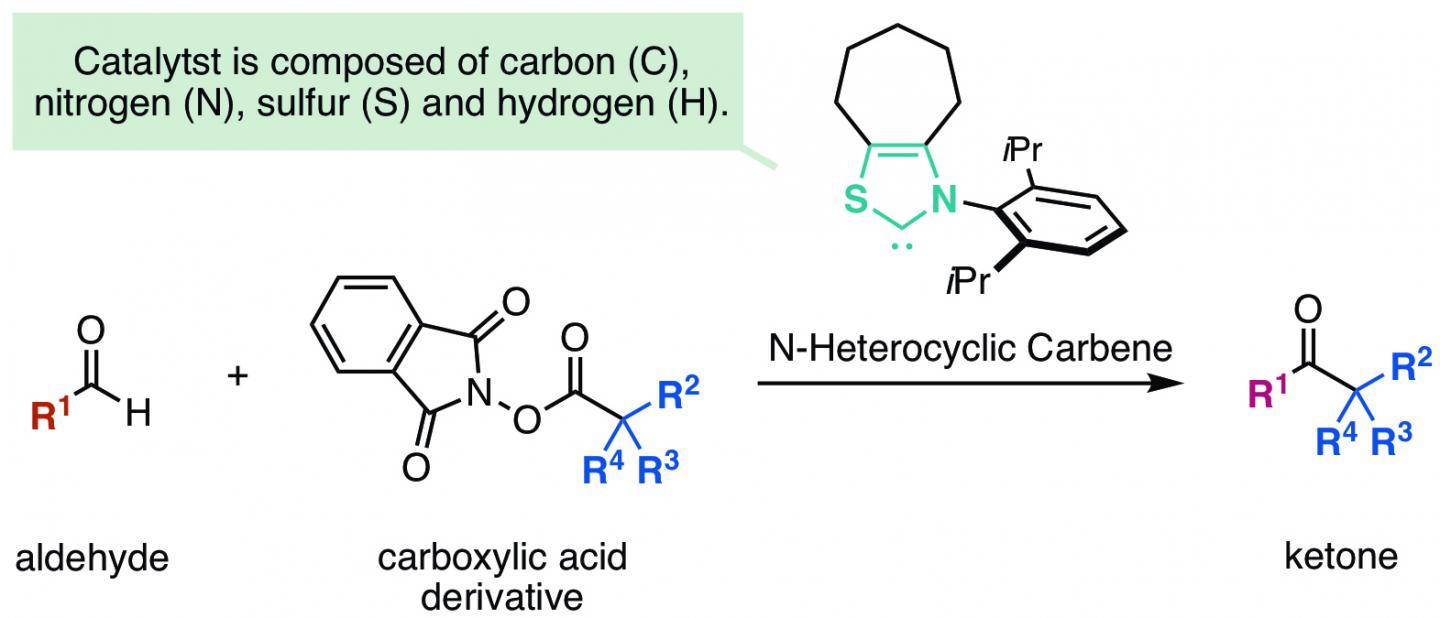
Ketones are important organic compounds found in a variety of pharmaceuticals, agrochemicals, and natural products. The synthesis of a ketone directly from a corresponding readily-available aldehyde would be ideal and has been successfully performed using a metal catalyst. However, metal catalysts are expensive and the metal remaining in the final products may cause problems. On the other hand, N-heterocyclic carbene*1), an organic compound, is known to act as an organic catalyst*2) for such synthesis. N-heterocyclic carbene reacts with an aldehyde to form a Breslow intermediate*3). This Breslow intermediate, acting as a nucleophile followed by two-electron transfer, reacts with various electrophiles to form a ketone. However, this carbon-carbon bond formation following two-electron transfer is known to be sterically hindered and N-heterocyclic carbene is known to react only with electrophiles with activated electrons.
[Outline of research results]
The group at Kanazawa University, including two students, has succeeded in synthesizing a ketone from an aldehyde and a carboxylic acid by using N-heterocyclic carbene catalyst (Figure 1).
The key to the success of the reaction developed in this study was the discovery of a new reaction process in which radical-radical coupling followed by carbon-carbon bond formation took place after one electron transfer occurred from a Breslow intermediate of an enolate*4) formed from an aldehyde and an N-heterocyclic carbene to an electrophile (Figure 2). Since a highly reactive radical is used in the process of the carbon-carbon bond formation, it is possible to introduce a sterically bulky alkyl substituent, which used to be difficult in such a reaction.
It addition, the group succeeded in converting a carboxylic acid, part of many pharmaceutical drugs and natural products, into a ketone by the method described here (Figure 3).
[Future prospects]
The group at Kanazawa University succeeded in synthesizing a ketone, an important skeleton of many pharmaceuticals, from a readily available aldehyde and a carboxylic acid. The method makes possible the rapid synthesis of ketones even with bulky substituents, as well as those derived from pharmaceuticals or natural products.
Accordingly, our method is expected to accelerate drug discovery.
From a purely scientific viewpoint, it should be noted that the new reaction process, i.e. a radical-radical coupling after one electron transfer occurring from a Breslow intermediate, will be a new design guideline for N-heterocyclic carbene catalytic reactions.
###
[Glossary]
*1) Carbene
A carbene is a twofold coordination chemical species containing a neutral carbon atom with only six valence electrons.
*2) Organic catalyst
A catalyst accelerates a specific chemical reaction with itself being unchanged. An organic catalyst is a low molecular weight chemical catalyst that consists of atoms of carbon, hydrogen, oxygen, nitrogen, sulfur, etc. but without metal elements.
*3) Breslow intermediate
A Breslow intermediate is a chemical species having an enol skeleton, being formed by a reaction of aldehyde and N-heterocyclic carbene. It is regarded as an acyl anion equivalent.
*4) Enolate
An enolate is an anionic chemical species with a deprotonated hydroxyl group attached to one carbon atom of carbon-carbon double bond.
Media Contact
Yuki Kashin
[email protected]
Related Journal Article
http://dx.




