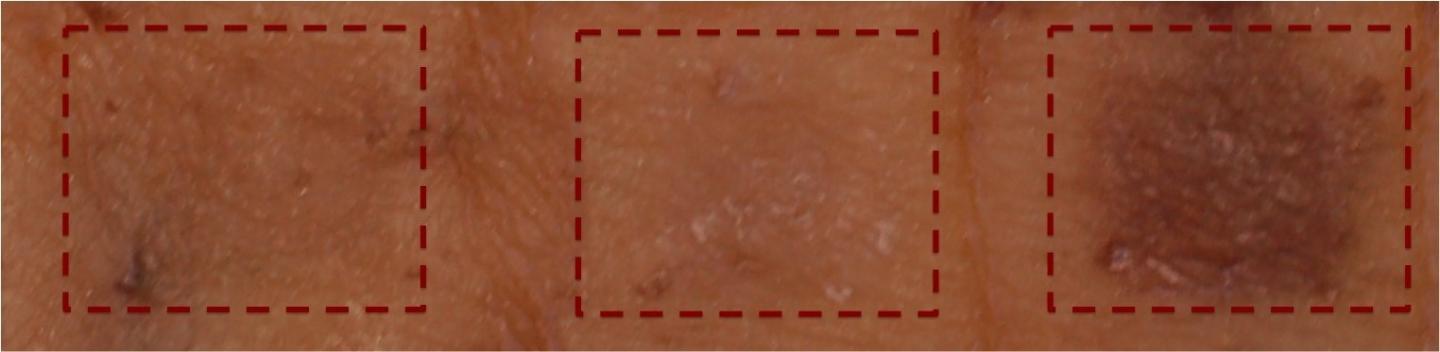
Credit: Nisma Mujahid and David E. Fisher, MD, PhD, Cutaneous Biology Research Center, Department of Dermatology, Massachusetts General Hospital
Investigators at Massachusetts General Hospital (MGH) and Dana-Farber Cancer Institute (DFCI) have developed a way of increasing pigmentation in human skin without the damaging effects of ultraviolet (UV) radiation. Their study, reported in the June 13 issue of Cell Reports, is a follow-up to a 2006 study that identified the molecular pathways underlying the tanning response and induced tanning in a strain of mouse that normally does not produce the protective, dark form of melanin.
"The activation of the tanning/pigmentation pathway by this new class of small molecules is physiologically identical to UV-induced pigmentation without the DNA-damaging effects of UV," says David E. Fisher, MD, PhD, chief of the Department of Dermatology at MGH who led both studies. "We need to conduct safety studies, which are always essential with potential new treatment compounds, and better understand the actions of these agents. But it's possible they may lead to new ways of protecting against UV-induced skin damage and cancer formation."
In the 2006 study published in Nature, Fisher's team — then based at DFCI — used a topical compound called forskolin to induce tanning in a strain of red-haired mice, in which a genetic variant interrupts the pathway leading to production of melanin pigment. Forskolin activates a protein further down the pigmentation pathway, bypassing the interruption and inducing production of the protective dark pigment called eumelanin.
Subsequent testing in human skin samples of forskolin and a related compound with similar action was not successful, probably because human skin is around five times thicker than the skin of mice. This led the investigators to explore a different approach. Enzymes called salt-inducible kinases (SIKs) were known to regulate transcription of a protein even further down the pigmentation pathway, and inhibiting SIK expression had been shown by a research group in Japan to activate pigmentation in mice.
Initial experiments with a previously described SIK inhibitor produced marked tanning in the same strain of red-haired mice used in the 2006 study. Skin darkening increased with daily treatment, becoming virtually black within a few days, and gradually decreased after application was halted, just as a normal tan does. When that compound proved to have limited activity in human skin samples, likely due to limited penetration, Fisher's group turned to colleagues in the DFCI laboratory of Nathanael Gray, PhD, a co-author of the current study.
A new class of small-molecule SIK inhibitors, developed by Gray's team and tested by Fisher's, was found to be better able to penetrate cultured human skin samples and induce significant darkening following eight days of daily, topical administration. Microscopic examination of the treated skin samples confirmed that eumelanin pigment was produced and deposited near the skin surface in patterns typical to what is seen with UV-induced pigmentation/tanning, suggesting activation of the same pigmentation pathway.
"We are excited about the possibility of inducing dark pigment production in human skin without a need for either systemic exposure to a drug or UV exposure to the skin," says Fisher, the Wigglesworth Professor of Dermatology at Harvard Medical School and director the MGH Cutaneous Biology Research Center (CBRC).
###
The co-lead authors of the Cell Reports paper are Nisma Mujahid, MGH Dermatology/CBRC, and Yanke Liang, DFCI Department of Cancer Biology. Additional co-authors are Ryo Murakami, Allison Dobry, Yusuke Suita, Qing Yu Weng, Jennifer Allouche, PhD, Lajos Kemeny, MD, Andrea Herman, and Elisabeth Roider, MD, MGH Dermatology/CBRC; and Hwan Geun Choi and Jinhua Wang, PhD, DFCI Cancer Biology. The study was supported by National Institutes of Health grants 5P01 CA163222 and 5R01 AR043369-19, the Melanoma Research Alliance, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation and Canadian Institutes of Health Research grant DFS-140391. A patent application covering the findings of this study has been jointly filed by the MGH and DFCI.
Massachusetts General Hospital, founded in 1811, is the original and largest teaching hospital of Harvard Medical School. The MGH Research Institute (http://www.massgeneral.org/research/about/RI-welcome.aspx) conducts the largest hospital-based research program in the nation, with an annual research budget of more than $800 million and major research centers in HIV/AIDS, cardiovascular research, cancer, computational and integrative biology, cutaneous biology, genomic medicine, medical imaging, neurodegenerative disorders, regenerative medicine, reproductive biology, systems biology, photomedicine and transplantation biology. The MGH topped the 2015 Nature Index list of health care organizations publishing in leading scientific journals and earned the prestigious 2015 Foster G. McGaw Prize for Excellence in Community Service. In August 2016 the MGH was once again named to the Honor Roll in the U.S. News & World Report list of "America's Best Hospitals.
Media Contact
Julie Cunningham
[email protected]
617-724-6433
@MassGeneralNews
http://www.mgh.harvard.edu
Related Journal Article
http://dx.doi.org/10.1016/j.celrep.2017.05.042
############
Story Source: Materials provided by Scienmag





