Johns Hopkins Medicine researchers have developed and tested a new imaging approach they say will accelerate imaging-based research in the lab by allowing investigators to capture images of blood vessels at different spatial scales. Tested in mouse tissues, the method, dubbed “VascuViz,” includes a quick-setting polymer mixture to fill blood vessels and make them visible in multiple imaging techniques. The approach enables researchers to visualize the structure of a tissue’s vasculature, which in conjunction with detailed mathematical models or complementary images of other tissue elements can clarify the complex role of blood flow in health and disease, say the researchers. The combined images of the blood vessels should not only enhance the study of the biology of diseases that involve abnormalities in blood flow, such as cancer and stroke, but also advance our understanding of the structures and functions of tissues throughout the body, they say.
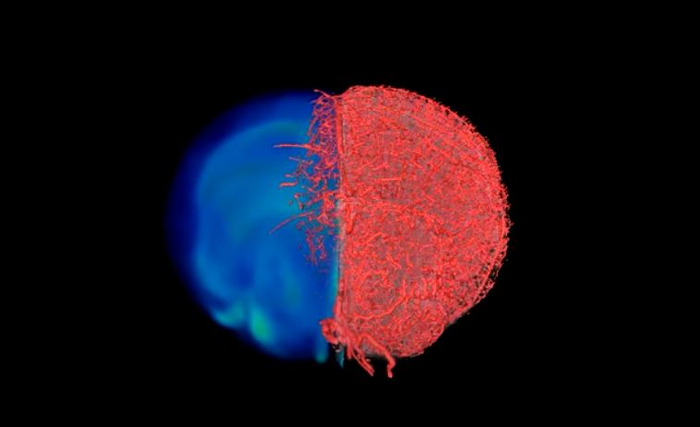
Credit: Credit: Johns Hopkins Medicine
Johns Hopkins Medicine researchers have developed and tested a new imaging approach they say will accelerate imaging-based research in the lab by allowing investigators to capture images of blood vessels at different spatial scales. Tested in mouse tissues, the method, dubbed “VascuViz,” includes a quick-setting polymer mixture to fill blood vessels and make them visible in multiple imaging techniques. The approach enables researchers to visualize the structure of a tissue’s vasculature, which in conjunction with detailed mathematical models or complementary images of other tissue elements can clarify the complex role of blood flow in health and disease, say the researchers. The combined images of the blood vessels should not only enhance the study of the biology of diseases that involve abnormalities in blood flow, such as cancer and stroke, but also advance our understanding of the structures and functions of tissues throughout the body, they say.
The report published Feb. 10 in Nature Methods.
“Usually, if you want to gather data on blood vessels in a given tissue and combine it with all of its surrounding context like the structure and the types of cells growing there, you have to re-label the tissue several times, acquire multiple images and piece together the complementary information,” says Arvind Pathak, Ph.D., professor of radiology, biomedical and electrical engineering and member of the Sidney Kimmel Comprehensive Cancer at the Johns Hopkins University School of Medicine. “This can be an expensive and time-consuming process that risks destroying the tissue’s architecture, precluding our ability to use the combined information in novel ways.”
Researchers use many different imaging methods, such as MRI, CT and microscopy to study the role of blood vessels in the lab. These images are useful for understanding the dynamics of how tissues develop disease or respond to treatment. However, integrating the data available in these images has remained a challenge because agents used to make a blood vessel visible to one imaging method can make it invisible on other tools. This limits the amount of data researchers can gather from a single sample.
VascuViz overcomes this problem by making the structure of the largest arteries to the smallest microvasculature visible to a variety of imaging tools, which allows researchers to develop a multilayered understanding of blood vessels and related tissue components with less time and effort.
The development of VascuViz is particularly useful in creating computerized visualizations of how complex biological systems such as the circulatory system work, and is a hallmark of the growing field of “image-based” vascular systems biology.
“Now, rather than using an approximation, we can more precisely estimate features like blood flow in actual blood vessels and combine it with complementary information, such as cell density,” says Akanksha Bhargava, Ph.D., postdoctoral fellow in the Pathak Lab within the Department of Radiology and Radiological Science at the Johns Hopkins University School of Medicine. To do this, VascuViz-based measurements are entered into computer simulations of blood flow, such as the cancer models Bhargava studies.
To create VascuViz, Bhargava tested several combinations of existing imaging agents and their suitability for different imaging methods. After multiple iterations, she found that a CT contrast agent named BriteVu and a fluorescently labeled MRI contrast agent called Galbumin-Rhodamine could be combined to create a compound that makes the macro- and microvasculature simultaneously visible when imaging with MRI, CT and optical imaging techniques without interference.
With the compound working in test tubes, the researchers then tested it in a variety of mouse tissues, perfusing it through the vascular system of breast cancer models, leg muscles, the brain and kidney tissues. The resulting images of the tissues acquired with MRI, CT and optical microscopy were then combined to create stunning 3D visualizations of the vasculature and associated components comprising these disease model and organ systems.
Due to VascuViz’s affordability and commercially available components, Pathak and his team hope it is globally adopted by scientists to help shed new light on different diseases involving the vasculature.
Other researchers involved in this study include Benjamin Monteagudo, Priyanka Kushwaha, Janaka Senarathna, Yunke Ren, Ryan Riddle and Manisha Aggarwal of the Johns Hopkins University School of Medicine.
This work was supported by the National Cancer Institute (51R01CA196701-05, 1R01CA237597-01A1), the National Institute of Dental & Craniofacial Research (5R01DE027957-02) and NIH Instrumentation grant (S10OD012287) and the Sidney Kimmel Comprehensive Cancer Center, Quantitative Sciences Pilot Project Grant.
The authors have no competing interests to declare.
To view a visual demonstration of the result of the VascuViz imaging pipeline, click here. Credit: Johns Hopkins Medicine.
Journal
Nature Methods
DOI
10.1038/s41592-021-01363-5