The human/mammalian brain is arguably the most complex biological system known. Even after witnessing massive progress in neuroscience, we are still far from a comprehensive understanding of mammalian brains and their pathologies. One important hurdle in our way to this goal is the high cost of neuroimaging systems, such as those used when studying animal models. For example, consider magnetic resonance imaging systems. While these devices provide exceptional three-dimensional spatial resolution, they also have huge purchase and maintenance costs, which limits their use in preclinical studies and basic scientific research. Similarly, most custom-built “mesoscopes,” which combine a decently sized field of view (FOV) with high spatial and temporal resolutions, rely on expensive CMOS cameras with a small pixel size.
Credit: Jose et al., doi 10.1117/1.NPh.11.1.014306.
The human/mammalian brain is arguably the most complex biological system known. Even after witnessing massive progress in neuroscience, we are still far from a comprehensive understanding of mammalian brains and their pathologies. One important hurdle in our way to this goal is the high cost of neuroimaging systems, such as those used when studying animal models. For example, consider magnetic resonance imaging systems. While these devices provide exceptional three-dimensional spatial resolution, they also have huge purchase and maintenance costs, which limits their use in preclinical studies and basic scientific research. Similarly, most custom-built “mesoscopes,” which combine a decently sized field of view (FOV) with high spatial and temporal resolutions, rely on expensive CMOS cameras with a small pixel size.
A research team from New Zealand decided to stand up to the challenge of making animal neuroimaging more accessible. Through a careful selection and combination of commercially available components, they built a custom low-cost mesoscope with attractive capabilities for imaging rodent brains. Their report is published in the Gold Open Access journal Neurophotonics.
One key feature of the proposed device is its modularity. The system consists entirely of “off-the-shelf” components, including an excitation source, a dichroic mirror, optical filters, lenses, and a CMOS camera, all arranged in what is known as an optical cage. Simply put, all elements are mounted onto a set of steel rods and rails that enable not only precise alignment of the optical path, but also freedom of movement and easy reconfiguration. Moreover, most of these elements are typically available in optics labs at accessible prices, making the entire system highly efficient and cost-effective.
Another key feature of the custom mesoscope is its reversible tandem lens configuration. This means that the objective lens (positioned close to the brain) and the imaging lens (positioned close to the camera) can be swapped. Using the lens with a larger focal length as the objective lens results in a larger FOV, which enables the user to see a larger area of the brain simultaneously at the cost of a lower resolution. On the other hand, the reverse configuration offers a smaller FOV but greater magnification and at higher resolution.
This feature makes the proposed mesoscope versatile and adaptable to different experimental requirements. “Reversing the configuration is straightforward and does not require optics alignment expertise,” highlights author Professor Frédérique Vanholsbeeck of the University of Auckland. “The researcher can easily change the order of the objective and imaging lenses to enable a smaller FOV during an experiment if they prefer improved resolution,” she explains.
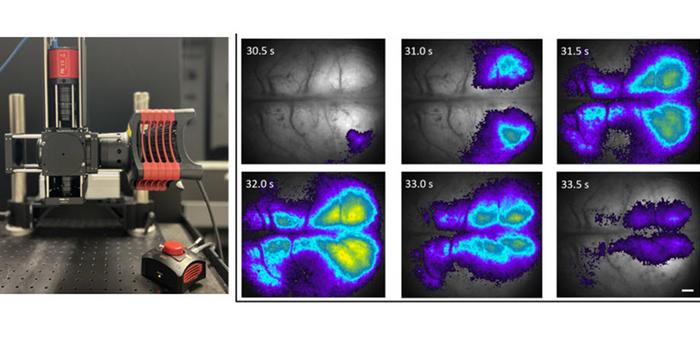
The research team thoroughly tested their system in a series of experiments involving in vitro imaging of rat brain slices and in vivo imaging of neonatal mice and rat brains. The in vitro experiments showed that the proposed mesoscope could capture crisp fluorescence images of brain sections, achieving single-cell resolution. On the other hand, the in vivo experiments showcased the capability of the system to visualize spontaneous neuronal activity in the cortex of mice brains when viewed through the skull, enabled through the use of a fluorescence calcium probe genetically introduced into the animals.
Overall, the mesoscope developed in this study could make neuroimaging more accessible to scientists everywhere, which could significantly accelerate progress in neuroscience and brain medicine. “Our custom-built reversible FOV mesoscope, which demonstrated excellent performance, is cost-effective; it was developed for under US$10,000,” remarks Prof. Vanholsbeeck, “The careful selection of components ensured its compactness, portability, and versatility, meaning that different types of samples and sample holders can be easily accommodated to enable a wide range of experiments both in vivo and in vitro.”
Hopefully, these efforts will serve as a stepping-stone to solving one of nature’s greatest mysteries—understanding how our brains work.
For details, see the original Gold Open Access article by A. Jose et al., “Low-cost reversible tandem lens mesoscope for brain imaging in rodents,” Neurophotonics 11(1), 014306 (2024), doi 10.1117/1.NPh.11.1.014306.
Journal
Neurophotonics
DOI
10.1117/1.NPh.11.1.014306
Article Title
Low-cost reversible tandem lens mesoscope for brain imaging in rodents
Article Publication Date
8-Mar-2024




