Understanding the ways our immune response changes as we age holds the key to designing better vaccines and boosting protection for people most at risk. Research published by Dr Michelle Linterman and her group today in Nature Immunology has explained that the organisation of the germinal centre, which is vital to the generation of longer-lived protection following vaccination, is altered in ageing. By demonstrating that these age-related changes can be reversed in mice, the research sets the foundation for interventions that bolster an effective vaccine response.
Credit: Babraham Institute
Understanding the ways our immune response changes as we age holds the key to designing better vaccines and boosting protection for people most at risk. Research published by Dr Michelle Linterman and her group today in Nature Immunology has explained that the organisation of the germinal centre, which is vital to the generation of longer-lived protection following vaccination, is altered in ageing. By demonstrating that these age-related changes can be reversed in mice, the research sets the foundation for interventions that bolster an effective vaccine response.
After a vaccination our immune system reacts by creating specialised structures called germinal centres that produce the immune cells (B cells) that provide long-term protection through the production of antibodies. Due to an age-dependent impairment in antibody production, older people have lower levels of protection from vaccination which also wanes more quickly compared to younger people. Protection by vaccination is essential to protect older people who become more susceptible to infections with age. Therefore, understanding how the age-related decline of the immune system can be reversed or mitigated is an important part of securing better health in later years.
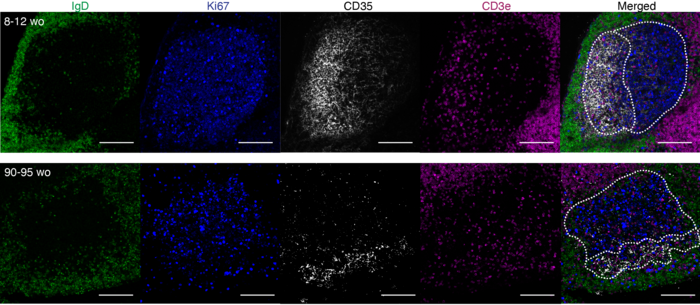
The correct function of the germinal centre response requires the coordination of cellular interactions across time and space. Germinal centres are made up of two distinct regions – the light zone and dark zone, with some cells located in specific areas, and others which move between the zones. B cells are shaped by their interactions in first the dark zone and then in the light zone.
Through a combination of mouse research, computer modelling and analysis of human vaccination data, the Linterman lab research team were able to show that changes to key interactors of B cells in the light zone of the germinal centre, T follicular helper cells, and also to light-zone specific cells called follicular dendritic cells (FDCs), were at the heart of the diminished vaccination response.
Dr Michelle Linterman, a group leader in the Institute’s Immunology programme, explains “In this study we looked at what was happening to different cell types in the germinal centre, particularly the structure and organisation of the germinal centre across its two functionally distinct zones, to try and understand what causes the reduced germinal centre response with age.
“What we found is that the T follicular helper cells aren’t where they should be and as a result, antibody-producing cells lose essential selection cues. Surprisingly we also uncovered an unknown role for T follicular helper cells in supporting the expansion of follicular dendritic cells in the light zone after vaccination.”
The team used 3D computer modelling to simulate the loss of Tfh cells from the light zone and a reduced FDC network, which recapitulate their findings and strengthened their hypothesis that these two factors were enough to be responsible for a suboptimal germinal centre response in aged mice.
Having identified the dependencies between the cell types, the researchers used genetically modified mice to control the location of Tfh cells in the germinal centre, demonstrating that the defective FDC response was caused by loss of Tfh from the light zone. Importantly, they were also able to correct the defective FDC response and boost the germinal centre response in aged mice by providing T cells that could correctly localise to the light zone.
The team also utilised data from human vaccination studies and found similar age-dependent changes in mice and humans.
“These findings give us a more complete picture of what the effects of age are on the germinal centre and vital insight into how we might address these in terms of developing effective strategies for enhancing vaccine response in older people” concluded Dr Linterman.
Journal
Nature Immunology
DOI
10.1038/s41590-023-01519-9
Method of Research
Experimental study
Article Title
Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging
Article Publication Date
22-May-2023




