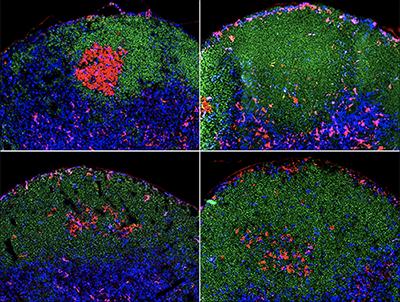
LA JOLLA, CA–Follicular helper T cells (Tfh cells), a rare type of T cells, are indispensable for the maturation of antibody-producing B cells. They promote the proliferation of B cells that produce highly selective antibodies against invading pathogens while weeding out those that generate potentially harmful ones. In their latest study, researchers at La Jolla Institute for Allergy and Immunology identified a key signal that drives the commitment of immature Tfh cells into fully functional Tfh cells and thus driving the step-by-step process that results in a precisely tailored and effective immune response.
Specifically, the study published in the May issue of Nature Immunology and led by La Jolla Institute Professor Shane Crotty, Ph.D., and Instructor Kok-Fai Kong, Ph.D., reported that an enzyme called TANK-binding kinase 1 (TBK1) controls the maturation of antibodies by associating with a key signaling molecule known as Inducible T cell Co-Stimulator or ICOS.
While ICOS was known to be clearly essential for most Tfh cell functions, the underlying mechanism had remained unclear. Understanding the molecular details of how ICOS exercises its critical influence may allow scientists to manipulate antibody specificity to design better vaccines and create novel treatments for autoimmune disorders. Autoimmunity lies at the root of over 80 disease including type 1 diabetes, multiple sclerosis, rheumatoid arthritis and lupus.
"Tfh cells have recently been recognized as important players in the immune system, and we now know they are essential for almost all antibody responses," said Crotty. "Tfh cells control the whole process of generating high affinity antibodies, and ICOS is a receptor molecule strongly required for Tfh cells to work. Understand ICOS better, and you can make more Tfh when needed, and block it when not needed."
In fact, TBK1 may also be a contributor to debilitating diseases such as ALS (Lou Gehrig's disease) and childhood herpes simplex virus encephalitis, if its connection with ICOS somehow triggers B cell activation and specific antibody production against the body's own cells in ALS or an excessive response to the invading viruses in childhood encephalitis. Knowing how ICOS is regulated will help scientists understand how these diseases erupt, as much as it could help further vaccine development.
ICOS gets antibody production started by promoting the maturation of Tfh cells, which migrate into compartments or follicles of immune organs such as spleens and lymph nodes, which themselves are full of B cells. The body has billions of B cells, each one and its daughter cells recognize something different, so very few of which can recognize the structure of any given germ. Once in contact with Tfh, some B cells become activated, migrate to an area of the follicle called the germinal center and start dividing rapidly under influence from Tfh. While most B cells have a low affinity for pathogens, which might effectively fend off a relatively mild virus, Tfh (with the stimulation of ICOS) allows the select few that produce highly specific and more strongly reactive antibodies to proliferate and outcompete their less specific brethren. The survivors undergo successive rounds of mutation resulting in better and better antibodies during the course of an immune response.
"We were looking to find out a specific signaling pathway that impacted ICOS," said Kong. "There is a family of such checkpoint molecules like ICOS, but ICOS has a capability that is not shared with other checkpoint molecules. Its ability to drive antibody differentiation is found only in ICOS, and not in other family members. The connection between ICOS and TBK1 provides important clues to this mystery."
Kong and Crotty's team discovered that ICOS recruits the signaling TBK1 enzyme, which activates certain genes in Tfh cells, thus beginning the whole process of high-affinity antibody production. The team led by Dr. Kong identified a region of the ICOS molecule that recruits TBK1, and moreover, disrupting this area prohibits the association between ICOS and TBK1 and severely impairs Tfh and ultimately antibody production. Therefore, this could provide an important target for disease interventions or vaccine designs.
The researchers are now looking at how ICOS signals can be altered to diminish autoimmune disorders and augmented for more effective vaccine development, and are beginning research on how ICOS signaling may benefit Chimeric Antigen Receptor-T cell (CAR-T) therapies, which involves engineering of patient's own immune cells to recognize and attack their cancers.
###
Other contributors included Christophe Pedros, Yaoyang Zhang, Joyce Hu, Youn Soo Choi, Ann Canonigo-Balancio, John Yates and Amnon Altman.
Link to full article: 10.1038/ni.3463
This work was supported by grants from the National Institutes of Health (CA35299, AI109976, AI063107, and AI072543), and Young Investigator Award #270056 from the Melanoma Research Alliance
About La Jolla Institute
La Jolla Institute for Allergy and Immunology is dedicated to understanding the intricacies and power of the immune system so that we may apply that knowledge to promote human health and prevent a wide range of diseases. Since its founding in 1988 as an independent, nonprofit research organization, the Institute has made numerous advances leading towards its goal: life without disease®.
Media Contact
Jessica Roi
[email protected]
858-752-6645
@liairesearch
http://www.liai.org
The post LJI scientists discover molecular mechanism for generating specific antibody responses appeared first on Scienmag.