Credit: Immanuel Wagner/imma.tv
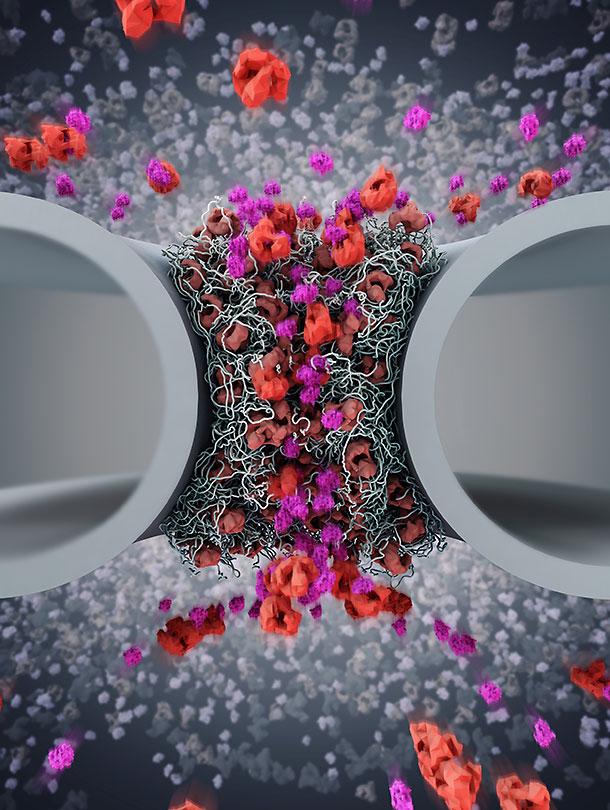
Nuclear pore complexes are tiny channels where the exchange of substances between the cell nucleus and the cytoplasm takes place. Scientists at the University of Basel report on startling new research that might overturn established models of nuclear transport regulation. Their study published in the Journal of Cell Biology reveals how shuttling proteins known as importins control the function of nuclear pores – as opposed to the view that nuclear pores control the shuttling of importins.
Genetic information is protected in the cell nucleus by a membrane that contains numerous nuclear pores. These pores facilitate the traffic of proteins known as importins that deliver molecular cargoes between the nucleus and the surrounding cytoplasm.
In contrast to prevailing views, the team led by Prof. Roderick Lim, Argovia Professor at the Biozentrum and the Swiss Nanoscience Institute of the University of Basel, has now demonstrated that the nuclear pore complex does not work like a simple filter that regulates the nuclear transport process. Rather, different importins cooperate to continuously open and close the pore like a "revolving door".
Importins regulate nuclear pores
For a long time scientists have reasoned that a molecular filter within the nuclear pore complex prevents or enables the passage of molecules into the nucleus. Lim's current study now shows that this filter alone is not sufficient for barrier function but provides only the basic infrastructure for establishing one. Instead, cargo-carrying importins function as bona fide components that regulate the nuclear pore complex transport barrier.
Moreover, Lim and colleagues show how the shuttling of importins is coupled to their barrier function. In fact, importins exist in two interacting forms: alpha and beta. Importin beta promotes cargo access into the pore whereas Importin alpha determines the cargo that can enter the nucleus.
Surprisingly, the team has now discovered that importin alpha acts as a molecular switch that helps to release or retain importin beta to open or close the pore. In the absence of importin alpha, importin beta loses its ability to shuttle through the nuclear pore channel.
Importins in health and disease
The insights provided by the study also have implications for the understanding of diseases associated with transport defects at the nuclear pore complex, such as cancer.
"We always thought of the nuclear pore complex as a standalone machine that controls nuclear transport", says Lim.
"Now, we have a much greater appreciation for how the systematic interplay of importin alpha and beta are able to regulate the nuclear pore complex to sustain continuous transport. Hence, if importin alpha malfunctions the revolving door mechanism might get stuck such that essential proteins cannot get to their nuclear destinations. Or if importin beta is defective, the pore might become leaky against unwanted substances that can enter and poison the nucleus."
###
Original article
Larisa E. Kapinos, Binlu Huang, Chantal Rencurel and Roderick Y.H. Lim
Karyopherins regulate nuclear pore complex barrier and transport function
Journal of Cell Biology (2017), doi: 10.1083/jcb.201702092
Media Contact
Cornelia Niggli
[email protected]
@UniBasel_en
http://www.unibas.ch/
Original Source
https://www.unibas.ch/en/News-Events/News/Uni-Research/Like-a-Revolving-Door.html http://dx.doi.org/10.1083/jcb.201702092