UNC-Chapel HIll scientist Charles Carter, PhD, published seminal work on the inner workings of chemical machines inside our cells that turn energy into action, the foundation of life
Credit: Carter, UNC School of Medicine
In the popular book The Demon in the Machine, physicist Paul Davies argues that what’s missing in the definition of life is how biological processes create “information,” and such information storage is the stuff of life, like a bird’s ability to navigate or a human’s ability to solve complex problems. The “Demon” Davies refers to is Maxwell’s Demon, as proposed by 19th century physicist James Clerk Maxwell as a thought experiment. Maxwell’s hypothetical “demon” controls a gate between two chambers of gas and knows when to open the gate only to allow gas molecules moving faster than average to pass through it. This way, a chamber could be heated and create “energy” to be put to work. Such a demon would amount to a workaround of the Second Law of Thermodynamics. And that, as we know, is impossible. We also know, of course, that demons don’t exist.
However, living things use many protein devices called enzymes that mimic such a demon each time a muscle contracts or when any chemical reaction needs to be driven uphill and away from thermodynamic equilibrium like the gas molecules chosen by the demon. How these dynamic machines work has long been puzzling. Over the past 75 years, scientists have chipped away at this problem without identifying precise details of how any of these enzyme machines accomplishes the sleight of hand that sustains living things, such as humans who live in a chemical state far from equilibrium.
For the first time, in a paper published in Proteins: Structure, Function, and Bioinformatics by Charlie Carter, PhD, professor in the Department of Biochemistry and Biophysics at the UNC School of Medicine, and supported by the National Institute of General Medical Sciences, describes the details that enable one such machine to work like Maxwell’s demon.
The machine in question is an enzyme called tryptophanyl-tRNA synthetase, or TrpRS, which can use the chemical energy stored in the universal fuel molecule – Adenosine triphosphate (ATP) – to ensure that whenever the sequence of any gene specifies tryptophan, the amino acid tryptophan is inserted into the sequence of linked amino acids that compose the translated protein. By assuring that the correct amino acid is selected, TrpRS therefore translates the genetic code for tryptophan when any of the tens of thousands of genes in human cells is translated into the corresponding protein. Translating the code into the amino acid sequence specified by the gene gives the newly created protein sequence the information telling it how to fold up and exert nanoscale control over some aspect of cellular chemistry.
Carter’s previous work with TrpRS led to a fundamental revision of how genetic coding began. In this latest paper, Carter investigates how TrpRS mimics Maxwell’s demon. The details he describes may represent a solution to the more general problem of how all energy in living things is transformed from fuel to useful work, such as muscles contracting, biosynthetic reactions that build new molecules required by the cell, or information managed by signaling networks driven by hydrolyzing a related fuel –Guanosine triphosphate (GTP)–that keep cellular chemistry under tight regulatory control.
TrpRS has several moving parts that identify tryptophan and attach it specifically to the correct transfer RNA if and only if the relative motions of certain flexible, changing parts of the protein called “domains” are tightly coupled to ATP hydrolysis. These domains are dynamic. How they bend and move is referred to as “domain motion.” Carter shows how domain motion in general and ATP hydrolysis both depend on the completion of the other.
Hydrolysis of ATP cannot happen unless the domain motion occurs, but the domain motion itself cannot occur unless ATP is hydrolyzed. Paradoxically, the two conditions, or “gates,” occur in coordination. Carter calls this two-way dependence “reciprocally-coupled gating.”
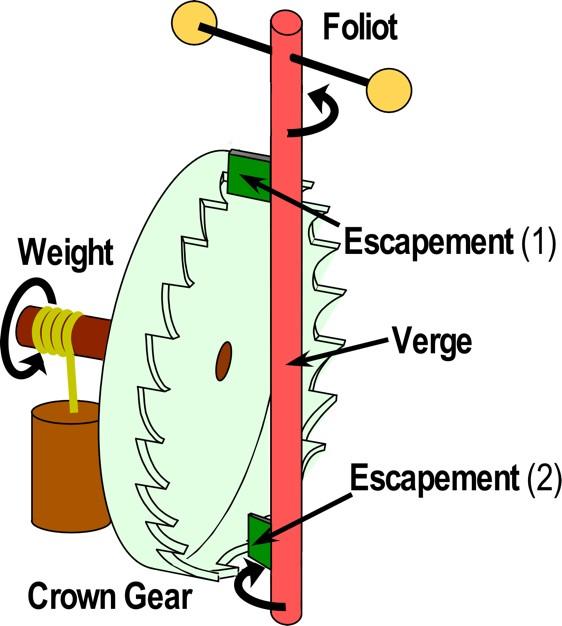
“This tight coupling is like the ‘escapement mechanism’ in a ticking mechanical clock (see figure),” Carter said. “The two kinds of gates function like the two green plates, each allowing the main “crown” gear to slip one gear at a time, but only in one direction, as the pendulum swings. This is how a clock converts the energy of unwinding the weight around the shaft of the crown gear, driving the pendulum into a time-keeping device.”
Scientists are increasingly recognizing escapement mechanisms as fundamental to all cellular processes driven by hydrolysis of fuel molecules like ATP and GTP. Carter’s work shows for the first time exactly how domain motions are efficiently coordinated with the consumption of the fuel. Notably, the GTPase superfamily also includes a high proportion of known oncogenes whose mutations make their escapement mechanisms malfunction sufficiently to cause cancer.
“It is likely that most or all of life’s motors and signaling devices that use either ATP or GTP will exhibit comparable gating mechanisms,” Carter said. “Scientists have known for 75 years that such mechanisms must exist. It is thrilling to uncover such a complete example of how gating mechanisms work together to ensure that we waste so little of the fuel we consume.”
###
Media Contact
Mark Derewicz
[email protected]
984-974-1915
Original Source
http://news.
Related Journal Article
http://dx.




