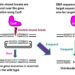
SAN FRANCISCO, CA—In recent years, scientists have used gene modification technologies to reprogram immune cells into therapeutics that can attack cancers. But such immunotherapies don’t work for all patients or all cancer types, and screening through every possible combination of genetic changes that might improve these reprogrammed immune cells is a daunting and slow task.

Credit: Michael Short/Gladstone Institutes
SAN FRANCISCO, CA—In recent years, scientists have used gene modification technologies to reprogram immune cells into therapeutics that can attack cancers. But such immunotherapies don’t work for all patients or all cancer types, and screening through every possible combination of genetic changes that might improve these reprogrammed immune cells is a daunting and slow task.
Now, scientists at Gladstone Institutes and UC San Francisco (UCSF) have developed a technology that lets them rapidly “snap” together thousands of different combinations of genetic edits to test in immune cells. They used their screening technology, called Modular Pooled Knockin Screening (ModPoKI), to identify a new combination of genes that, when added to immune cells, makes the cells last longer and become more effective at fighting cancers.
“This is a major step forward in our ability to ask questions about how we put pieces of genetic programs together into cells and test how they may be advantageous for patients,” says Alex Marson, MD, PhD, director of the Gladstone-UCSF Institute of Genomic Immunology and senior author of the new study published in Cell. “I think this is going to accelerate the development of better cellular therapies.”
“This study demonstrates the power of using high-throughput genomics to discover and engineer novel molecular programs in cell therapies, and further, to understand the impact of these programs on the T cell state that is required for cancer killing,” adds Ansuman Satpathy, MD, PhD, affiliate investigator at Gladstone, assistant professor in the Department of Pathology at the Stanford School of Medicine, and co-author of the study.
Mixing and Matching Parts
The immune system’s T cells play a key role in fighting cancer. When a T cell recognizes a cancer cell as foreign, through a receptor on its surface, it targets the cancer cell for destruction. Scientists have discovered how to alter receptor proteins on T cells—often done by adding DNA sequences for an artificial chimeric antigen receptor (CAR)—so the T cells can more easily recognize and eliminate cancer cells.
With CAR T-cell therapy, cancer patients’ T cells are removed with a blood draw, reprogrammed genetically in the lab to introduce these new CAR receptors, and infused back into the patients’ blood. Many CAR T-cell therapies, however, still have limitations: they often don’t work against solid tumors, they can wear out over time, and some CARs don’t elicit strong enough immune responses to kill cancer cells.
“CAR T cells have been incredibly successful in the treatment of blood cancers like leukemia and lymphoma, but we’re still searching for ways to optimize them and apply them to other cancers,” says Franziska Blaeschke, MD, PhD, a postdoctoral fellow in Marson’s lab and first author of the new paper. “Until now, what we’ve been lacking is a systematic way to discover what genetic changes in a T cell will be most effective at improving CAR T cells.”
To fill that gap, the team of scientists developed ModPoKI. The technology combines multiple genes into long stretches of DNA for use in a CRISPR gene editing platform. The group used this tool to create approximately 10,000 possible combinations of these DNA stretches by mixing hundreds of genes with DNA for a specific CAR. Then, they pasted the stitched-together DNA sequences into a defined location in the T cell genome using CRISPR.
Each T cell took up a different DNA sequence, and the researchers then raced the cells against each other to see which ones performed best in various tests that could predict anti-tumor activity. An easy-to-read DNA barcode on each ModPoKI-generated set of genes let them track which gene combination led to improved T cells.
The Lego-like ability to combine genes in new ways before adding them to the cells is what allowed them to rapidly discover combinations that might improve CAR T cells—without having to manually choose and engineer each combination.
“Rather than having to make individual guesses about what might improve the function of cells and work through them one by one, we can click these pieces together and very rapidly test many of them in succession,” says Theodore Roth, MD, PhD, a former member of Marson’s lab now working with Satpathy’s group, and co-senior author of the study. “It’s a really useful piece of molecular biology.”
Toward Better Therapeutics
The genes that Marson’s team added to cells in the new study were surface receptors (both natural and engineered receptors designed to send boosting signals to CAR T cells) and transcription factors (which turn other genes on and off). When they analyzed test results from the hundreds of surface receptors and transcription factors, the researchers discovered that different CARs can be optimized by different factors.
“It turns out it’s not a one-transcription-factor-fits-all approach,” says Blaeschke. “So, when researchers are developing new CAR T cells, it makes sense to check what other factors will be best to engineer at the same time. Our work produced a kind of atlas scientists can use to combine different transcription factors with different T cell receptors or CARs.”
They also pinpointed a combination of two transcription factors that often seemed to improve CAR T cells. These two transcription factors—known as BATF and TFAP4—boosted the fitness of a CAR T cell that had previously been developed to treat childhood brain tumors.
“In the lab, the ModPoKI sequence with BATF and TFAP4 made CAR T cells show potential to improve anti-tumor activity,” says Marson. “Next, we need to do more work to determine whether adding these transcription factors will make CAR T cells more effective in human cancer patients.”
###
About the Study
The paper, “Modular Pooled Discovery of Synthetic Knockin Sequences to Program Durable Cell Therapies,” was published in the journal Cell on September 14, 2023. Other authors are: Yan Yi Chen, Ryan Apathy, Bence Daniel, Andy Y. Chen, Peixin Amy Chen, Katalin Sandor, Wenxi Zhang, Zhongmei Li, Cody Mowery, Tori N. Yamamoto, William A. Nyberg, Angela To, Ruby Yu, Ralf Schmidt, and Daniel B. Goodman of Gladstone; Raymund Bueno, Min Cheol Kim, Justin Eyquem, Julia Carnevale, Eric Shifrut, and Chun Jimmie Ye of UCSF; and Tobias Feuchtinger of Ludwig Maximilian University of Munich.
The work was supported by the National Institutes of Health (S10 RR028962, P30 DK063720, S10 1S10OD021822-01, F30DK120213), the National Cancer Institute (1R01CA276368-01, 5T32CA108462, 1K08CA252605-01), the Parker Institute for Cancer Immunotherapy, the Cancer League, the James B. Pendleton Charitable Trust, the UCSF Medical Scientist Training Program (T32GM007618), UCSF Endocrinology Training Grant (T32 DK007418), the Burroughs Wellcome Fund, the Lydia Preisler Shorenstein Donor Advised Fund, the Care-for-Rare Foundation, the German Research Foundation (SFB-TRR338/1 2021 –452881907), an Emerging Investigators EHA-EBMT Joint Fellowship Award, Lloyd J. Old STAR Awards (Cancer Research Institute), a Pew-Stewart Scholars in Cancer Research Award, the Simons Foundation, Innovative Genomics Institute, the Chan Zuckerberg Biohub, the Byers family, Barbara Bakar, Karen Jordan, Elena Radutzky, the Austrian Exchange Service, the Austrian Society of Laboratory Medicine, and the Max Kade Foundation.
About Gladstone Institutes
Gladstone Institutes is an independent, nonprofit life science research organization that uses visionary science and technology to overcome disease. Established in 1979, it is located in the epicenter of biomedical and technological innovation, in the Mission Bay neighborhood of San Francisco. Gladstone has created a research model that disrupts how science is done, funds big ideas, and attracts the brightest minds.
About UCSF
The University of California, San Francisco (UCSF) is exclusively focused on the health sciences and is dedicated to promoting health worldwide through advanced biomedical research, graduate-level education in the life sciences and health professions, and excellence in patient care. UCSF Health, which serves as UCSF’s primary academic medical center, includes top-ranked specialty hospitals and other clinical programs, and has affiliations throughout the Bay Area. Learn more at ucsf.edu, or see our Fact Sheet.
Journal
Cell
DOI
10.1016/j.cell.2023.08.013
Article Title
Modular pooled discovery of synthetic knockin sequences to program durable cell therapies
Article Publication Date
14-Sep-2023