Preeclampsia remains a daunting challenge in obstetrics, characterized by hypertension and organ dysfunction that jeopardize both maternal and fetal health globally. Despite advances in prenatal care, the underlying molecular mechanisms have eluded complete understanding, constraining the development of effective therapies. In a groundbreaking study published in Nature Communications, researchers have elucidated the integral role of the LAT1-NRF2 signaling axis in modulating the pathological imbalance of angiogenic factors and oxidative stress characteristic of preeclampsia, forging a novel pathway toward potential therapeutic intervention.
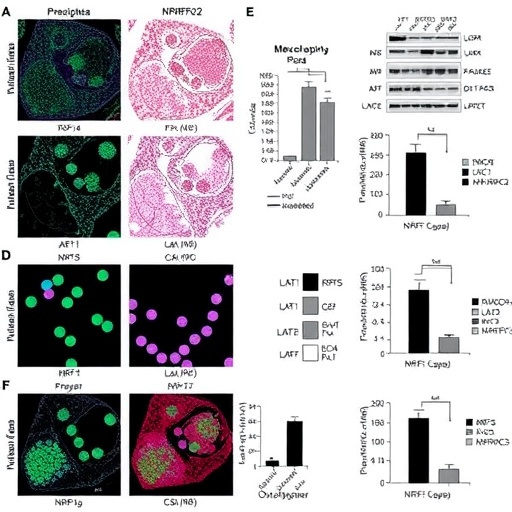
The study delves into the dysregulated balance between soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF), a hallmark of preeclampsia. Elevated sFlt-1 sequesters vascular endothelial growth factor (VEGF) and PlGF, impairing angiogenesis and endothelial function, which precipitates systemic vascular dysfunction. The researchers reveal that the LAT1 (L-type amino acid transporter 1) coupled with the NRF2 (nuclear factor erythroid 2-related factor 2) transcription factor forms a critical regulatory axis that governs this angiogenic imbalance by modulating oxidative stress within the placenta.
LAT1 is known primarily for its role in amino acid transport across membranes, crucial for cellular metabolism and growth. Interestingly, the study uncovers that LAT1 expression is markedly upregulated in placental tissues from preeclamptic pregnancies. This upregulation appears to trigger downstream activation of NRF2, a master regulator of antioxidant responses. NRF2 activation orchestrates a transcriptional program aimed at counteracting oxidative damage, but paradoxically in preeclampsia, this response becomes maladaptive, contributing to the pathological milieu by improperly regulating sFlt-1 and PlGF levels.
The researchers employed a multifaceted approach combining molecular biology, biochemistry, and clinical sample analyses to parse this complex signaling cascade. Using placental explant cultures and trophoblast cell lines, they demonstrated that inhibition of LAT1 significantly suppressed NRF2 activation, leading to a normalization of the sFlt-1/PlGF ratio. Conversely, stimulation of LAT1 amplified oxidative stress markers and exacerbated the angiogenic imbalance. These in vitro findings were corroborated in vivo using preeclampsia mouse models, where pharmacological modulation of LAT1 improved vascular outcomes and reduced maternal hypertension.
A central revelation is how oxidative stress, classically viewed as a damaging byproduct, functions within this network as a signaling entity modulating angiogenic factors. NRF2 ordinarily acts as a sentinel mitigating oxidative insult, but the study shows that in preeclampsia, continuous LAT1-driven NRF2 activation disrupts delicate homeostasis, leading to persistent overproduction of sFlt-1 and suppression of PlGF. This unraveling of normal feedback loops crystallizes the notion that the LAT1-NRF2 axis is not merely a passive responder but an active driver of disease pathology.
Further genomic analyses revealed differential expression of downstream NRF2 target genes associated with redox balance and inflammation within the placenta. This altered transcriptional landscape underscores a broader systemic effect where chronic oxidative stress and inflammation intertwine, aggravating endothelial dysfunction and promoting hypertension. Intriguingly, LAT1-NRF2 signaling also impacts mitochondrial function, a pivotal factor in cellular energetic homeostasis and reactive oxygen species generation, compounding placental insufficiency.
Beyond its fundamental mechanistic insights, the study proposes therapeutic avenues targeting LAT1 as a means to recalibrate the sFlt-1/PlGF axis and ameliorate oxidative damage. Preclinical intervention with LAT1 inhibitors demonstrated promising efficacy in restoring angiogenic equilibrium and reducing hypertensive parameters in animal models. These findings pave the way for clinical trials exploring such interventions, which could revolutionize management strategies for preeclamptic women who currently face limited treatment options predominantly focused on symptom management rather than root causes.
Moreover, the identification of the LAT1-NRF2 axis provides a potential biomarker axis for early detection and stratification of preeclampsia severity. Measurement of LAT1 expression or activity could enhance predictive accuracy when combined with existing assays of sFlt-1 and PlGF levels, potentially allowing for precise, timely clinical decision-making. This has significant implications for improving prenatal care outcomes and reducing maternal-fetal morbidity.
The broader implications of this research extend to other oxidative stress-related pathologies where angiogenic dysregulation is implicated. The mechanistic paradigm articulated here may inform studies into cardiovascular diseases, cancer, and chronic inflammation, where LAT1 and NRF2 pathways are similarly dysregulated. This cross-disease relevance underscores the study’s profound impact, heralding further investigations into amino acid transporters as pivotal molecular nodes in human disease.
Importantly, the authors highlight the dynamic interplay between metabolic pathways and redox signaling as a fertile ground for future research. LAT1’s role as more than a mere transporter, acting instead as a sensor and modulator of cellular stress responses, challenges traditional compartmentalized views of placental physiology. This paradigm shift could inspire novel diagnostic and therapeutic toolkits that leverage metabolic modulators to fine-tune placental and vascular health.
As the scientific community digests these insights, questions remain about the nuances of LAT1-NRF2 regulation and its interaction with other signaling networks in the placenta. Elucidating the upstream triggers that elevate LAT1 expression and decoding the temporal sequence of NRF2 activation could further refine understanding. Additionally, exploring patient heterogeneity and genetic predispositions influencing this axis could tailor personalized therapeutic approaches.
Notwithstanding these open questions, this study constitutes a milestone in unraveling preeclampsia’s molecular etiology. It moves the needle beyond descriptive pathology into actionable molecular targeting, opening a promising horizon for a condition long plagued by therapeutic challenges. The potential to intervene at a nodal point governing both angiogenesis and oxidative stress offers hope for breakthroughs that can save lives and improve pregnancy outcomes globally.
In sum, the identification of the LAT1-NRF2 axis as a master regulator of sFlt-1/PlGF imbalance and oxidative stress in preeclampsia represents a pivotal advance. It integrates metabolic, redox, and angiogenic signaling into a cohesive framework explicating preeclamptic pathology and provides a scaffold for innovative clinical modalities. As research progresses, this nexus will undoubtedly be a focal point of translational efforts aiming to conquer a condition that continues to exact a heavy toll on maternal and neonatal health worldwide.
Subject of Research: The molecular mechanisms underlying preeclampsia, focusing on the LAT1-NRF2 signaling axis and its regulation of the sFlt-1/PlGF imbalance and oxidative stress in placental pathology.
Article Title: LAT1-NRF2 axis controls sFlt-1/PlGF imbalance and oxidative stress in preeclampsia.
Article References:
Granitzer, S., Widhalm, R., Ellinger, I. et al. LAT1-NRF2 axis controls sFlt-1/PlGF imbalance and oxidative stress in preeclampsia. Nat Commun 16, 9112 (2025). https://doi.org/10.1038/s41467-025-64160-0
Image Credits: AI Generated
Tags: amino acid transport in placental healthangiogenic factors imbalanceendothelial dysfunction in pregnancyLAT1-NRF2 signaling axismaternal-fetal health challengesmolecular mechanisms of preeclampsiaoxidative stress in preeclampsiaplacental growth factor regulationpreeclampsia biomarkerssoluble fms-like tyrosine kinase-1therapeutic interventions for preeclampsiavascular dysfunction in pregnancy