Investigators at Johns Hopkins report they have developed human induced-pluripotent stem cells (iPSCs) capable of repairing damaged retinal vascular tissue in mice. The stem cells, derived from human umbilical cord-blood and coaxed into an embryonic-like state, were grown without the conventional use of viruses, which can mutate genes and initiate cancers, according to the scientists. Their safer method of growing the cells has drawn increased support among scientists, they say, and paves the way for a stem cell bank of cord-blood derived iPSCs to advance regenerative medicine research.
In a report published Jan. 20 in the journal Circulation, stem cell biologist Elias Zambidis, M.D., Ph.D., and his colleagues describe laboratory experiments with these non-viral, human retinal iPSCs, created using the virus-free method Zambidis first reported in 2011.
“We began with stem cells taken from cord-blood, which have fewer acquired mutations and little, if any, epigenetic memory, which cells accumulate as time goes on,” says Zambidis, associate professor of oncology and pediatrics at the Johns Hopkins Institute for Cell Engineering and the Kimmel Cancer Center. The scientists converted these cells to a status last experienced when they were part of six-day-old embryos.
Instead of using viruses to deliver a gene package to the cells to turn on processes that convert the cells back to stem cell states, Zambidis and his team used plasmids, rings of DNA that replicate briefly inside cells and then degrade.
Next, the scientists identified high-quality, multipotent, vascular stem cells generated from these iPSC that can make a type of blood vessel-rich tissue necessary for repairing retinal and other human material. They identified these cells by looking for cell surface proteins called CD31 and CD146. Zambidis says that they were able to create twice as many well-functioning vascular stem cells as compared with iPSCs made with other methods, and, “more importantly these cells engrafted and integrated into functioning blood vessels in damaged mouse retina.”
Working with Gerard Lutty, Ph.D., and his team at Johns Hopkins’ Wilmer Eye Institute, Zambidis’ team injected the newly derived iPSCs into mice with damaged retinas, the light-sensitive part of the eyeball. Injections were given in the eye, the sinus cavity near the eye or into a tail vein. When the scientists took images of the mice retinas, they found that the iPSCs, regardless of injection location, engrafted and repaired blood vessel structures in the retina.
“The blood vessels enlarged like a balloon in each of the locations where the iPSCs engrafted,” says Zambidis. The scientists said their cord blood-derived iPSCs compared very well with the ability of human embryonic-derived iPSCs to repair retinal damage.
Zambidis says there are plans to conduct additional experiments of their cells in diabetic rats, whose conditions more closely resemble human vascular damage to the retina than the mouse model used for the current study, he says.
With mounting requests from other laboratories, Zambidis says he frequently shares his cord blood-derived iPSC with other scientists. “The popular belief that iPSCs therapies need to be specific to individual patients may not be the case,” says Zambidis. He points to recent success of partially matched bone marrow transplants in humans, shown to be equally as effective as fully matched transplants.
“Support is growing for building a large bank of iPSCs that scientists around the world can access,” says Zambidis, although large resources and intense quality- control would be needed for such a feat. However, Japanese scientists led by stem-cell pioneer Shinya Yamanaka are doing exactly that, he says, creating a bank of stem cells derived from cord-blood samples from Japanese blood banks.
Experiments published in Zambidis’ Circulation article were funded by grants from the Maryland Stem Cell Research Fund, the National Institutes of Health’s National Heart, Lung and Blood Institute (HL099775, HL100397), National Eye Institute (EY09357), National Cancer Institute (CA60441); and Research to Prevent Blindness.
Under a licensing agreement between Life Technologies and the Johns Hopkins University, Zambidis is entitled to a share of royalties received by the University for licensing of stem cells. The terms of this arrangement are managed by Johns Hopkins University in accordance with its conflict-of-interest policies.
Scientists contributing to the research include Tea Soon Park, Imran Bhutto, Ludovic Zimmerlin, Jeffrey Huo, Pratik Nagaria, Connie Talbot, Jack Auilar, Rhonda Grebe, Carol Merges, and Gerard Lutty from Johns Hopkins; Diana Miller, Ricardo Feldman and Reyruz Rassool from the University of Maryland School of Medicine; Abdul Jalil Rufaihah, Renee Reijo-Pera, and John Cooke from Stanford University.
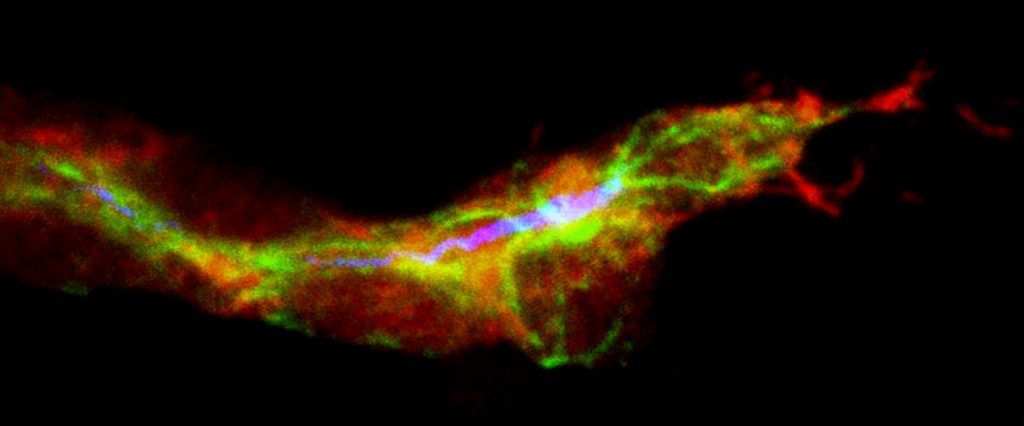
*Available upon request is an image of iPSC-derived vascular stem cells incorporating into a damaged retinal blood vessel and repairing it.
Story Source:
The above story is based on materials provided by Johns Hopkins Medicine.