In recent years, the alarming rise of antibiotic-resistant bacteria has posed a significant challenge to modern medicine, leading researchers to explore alternative treatment strategies. One promising avenue of research is the use of bacteriophages, particularly jumbo-sized bacteriophages, as therapeutic agents against multidrug-resistant organisms. The study by Paranos et al. sheds light on the use of a specific jumbo bacteriophage in treating infections caused by metallo-β-lactamase producing Pseudomonas aeruginosa, a notorious pathogen associated with severe healthcare-associated infections. This bacterium is well-known for its ability to resist a wide range of antibiotics, making it a primary concern in clinical settings.
Bacteriophages, or phages for short, are viruses that specifically infect bacteria. They exist in a variety of shapes and sizes, and among them, jumbo bacteriophages are particularly noteworthy due to their larger genomes and unique characteristics. These phages have gained attention for their potential therapeutic applications, especially in the face of increasing antibiotic resistance. The research conducted by Paranos and colleagues demonstrates not only the effectiveness of these phages but also showcases the potential of tailoring phage therapy to target specific bacterial pathogens.
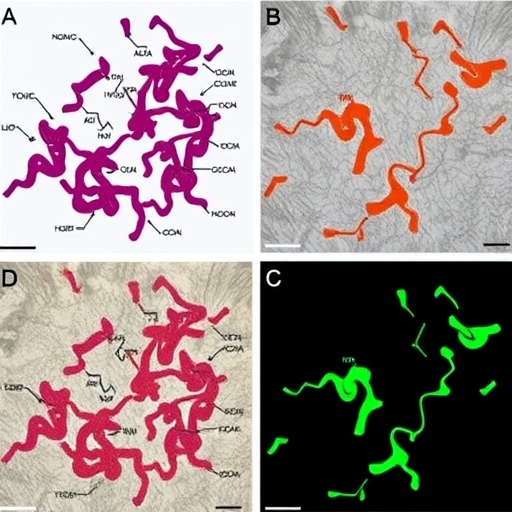
In their study, the researchers isolated a jumbo bacteriophage from environmental samples and evaluated its ability to infect and lyse metallo-β-lactamase producing Pseudomonas aeruginosa strains. This was particularly significant given the bacterium’s notorious resilience and its role in various critical infections, particularly in immunocompromised patients. The isolation process involved sophisticated techniques to ensure the specific targeting of Pseudomonas aeruginosa while avoiding non-target bacterial species, emphasizing the precision of phage therapy.
Phage therapy operates on the principle of using the lytic cycle of phages to eliminate bacterial infections. Upon successful attachment to a bacterial cell, these phages inject their genetic material, hijacking the bacterial machinery to produce new phage particles, leading to the eventual lysis and death of the bacterial cell. This method not only selectively destroys the targeted bacteria but also spares the beneficial microbes residing within the human microbiome—a crucial factor when considering the overall health and recovery of patients undergoing such treatments.
The study’s results demonstrated that the jumbo bacteriophage was effective in lysing clinical isolates of metallo-β-lactamase producing Pseudomonas aeruginosa in vitro. This effectiveness was indicative of the phage’s strong affinity for its bacterial host and its ability to disrupt the unique defense mechanisms that the bacterium employs against conventional antibiotics. The promise displayed in vitro paved the way for further investigation into the phage’s therapeutic potential in vivo.
In vivomodels were employed to assess the efficacy of the bacteriophage therapy in treating established infections in animals. The findings revealed a marked reduction in bacterial load and improved survival rates among treated subjects compared to the control group, affirming the bacteriophage’s therapeutic utility. These results highlight the potential of phage therapy as a viable alternative or adjunct to conventional antibiotic treatments, particularly in cases where standard therapies fail due to antibiotic resistance.
Moreover, the research underscores the importance of personalized medicine in treating complex infections. Since bacteriophages can be screened and selected for their specific activity against certain bacterial strains, treatments can be tailored to the unique bacterial profile of an individual patient. This targeted approach not only improves treatment outcomes but also minimizes the likelihood of adverse effects associated with broad-spectrum antibiotics.
The implications of successfully applying bacteriophage therapy extend beyond individual patient recovery. There is potential for significant public health benefits, especially as antibiotic resistance continues to escalate worldwide. By revitalizing interest in bacteriophages, healthcare systems may find a sustainable solution to combating resistant infections, improving health outcomes while altering the approach to infectious disease management.
The study also raises critical questions about the regulatory pathway for bacteriophage therapies. As these therapies transition from laboratory research to clinical applications, understanding the regulatory landscape and ensuring safety and efficacy will be paramount. The authors call for collaboration among scientists, healthcare providers, and regulatory agencies to establish clear guidelines that facilitate the responsible development and use of phage therapies in clinical practice.
Additionally, the research brings to light the importance of public awareness and education regarding antibiotic resistance and the potential role of phage therapy in addressing this global health crisis. As healthcare professionals, researchers, and advocates for patient care, it is essential to disseminate information about the advantages and mechanisms of phage therapy to foster acceptance among both practitioners and patients.
In summary, the work of Paranos and colleagues represents a significant step forward in the field of bacteriophage therapy, opening new doors for treatment options against metallo-β-lactamase producing Pseudomonas aeruginosa. The use of jumbo bacteriophages not only illustrates the adaptability and potential of phage therapy but also highlights the urgent need for innovative solutions to combat antibiotic resistance. As research continues to evolve in this promising field, the prospect of bacteriophage therapy becoming a mainstream approach in managing resistant bacterial infections looms large—a beacon of hope in the fight against antimicrobial resistance.
The convergence of bacteriophage research and clinical application suggests that the future of infectious disease treatment may rise from shadows cast by antibiotic resistance. With ongoing studies like that of Paranos et al., we are not only peering into the past and present of our battle with bacterial pathogens but also illuminating potential pathways towards a more effective and sustainable future in combating infection.
Subject of Research: The therapeutic application of jumbo bacteriophages against metallo-β-lactamase producing Pseudomonas aeruginosa clinical isolates.
Article Title: Therapeutic application of a jumbo bacteriophage against metallo-β-lactamase producing Pseudomonas aeruginosa clinical isolates.
Article References:
Paranos, P., Skliros, D., Zrelovs, N. et al. Therapeutic application of a jumbo bacteriophage against metallo-β-lactamase producing Pseudomonas aeruginosa clinical isolates.
J Biomed Sci 32, 74 (2025). https://doi.org/10.1186/s12929-025-01169-z
Image Credits: AI Generated
DOI: 10.1186/s12929-025-01169-z
Keywords: Bacteriophage therapy, Pseudomonas aeruginosa, antibiotic resistance, metallo-β-lactamase, jumbo bacteriophages, personalized medicine, infectious diseases.
Tags: antibiotic-resistant bacteria treatmentbacteriophage research studiescombating antibiotic resistanceenvironmental isolation of phageshealthcare-associated infections solutionsinnovations in infection controljumbo bacteriophage therapymetallo-β-lactamase resistancephage therapy against drug resistancePseudomonas aeruginosa infectionstargeting multidrug-resistant pathogenstherapeutic applications of phages