The Hong Kong University of Science and Technology (HKUST) and The Chinese University of Hong Kong (CUHK) today unveiled ground-breaking research that sheds light on how COVID-19 infection may lead to late pregnancy complications. The study reveals significant alterations in gene regulation within the placenta, paving the way for the development of potential molecular targets in future treatments to mitigate the detrimental effects of COVID-19 on maternal and fetal health.
Credit: HKUST
The Hong Kong University of Science and Technology (HKUST) and The Chinese University of Hong Kong (CUHK) today unveiled ground-breaking research that sheds light on how COVID-19 infection may lead to late pregnancy complications. The study reveals significant alterations in gene regulation within the placenta, paving the way for the development of potential molecular targets in future treatments to mitigate the detrimental effects of COVID-19 on maternal and fetal health.
Prior research involving 2,219 pregnant women with SARS-CoV-2 infection from various countries or regions, including Hong Kong1, has demonstrated that SARS-CoV-2 infection increases the risk of pregnancy-related death, severe maternal morbidities, and adverse fetal and newborn outcomes. In another study, a higher prevalence of preterm birth has been observed in pregnant women with SARS-CoV-2 infection. This increased risk is particularly pronounced among those who contract the virus during their third-trimester2, as compared to those who remain uninfected during pregnancy. Furthermore, a total of 142,561 cases of pregnant women with COVID-19 were reported in the United States between April 2020 and December 2022. The findings revealed that 11.21% of their infants born to these mothers were delivered preterm, and 9.7% required admission to the neonatal intensive care unit3. Despite these findings, the underlying molecular mechanisms driving the increased risk of adverse pregnancy outcomes associated with COVID-19 remain to be fully understood.
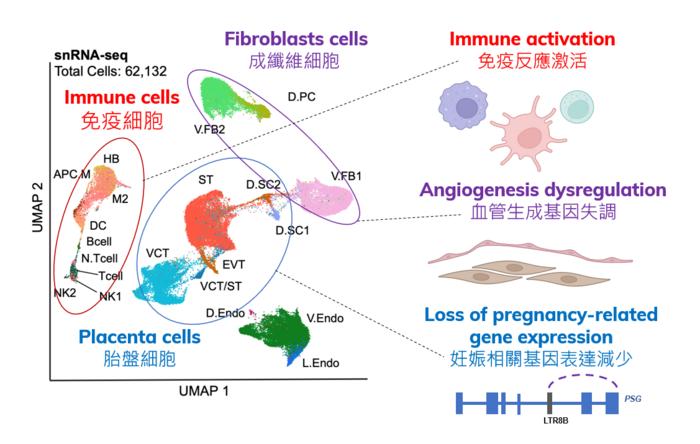
The research team, led by Prof. Danny LEUNG, Associate Professor of the Division of Life Science and Director of the Center for Epigenomics Research at HKUST, and Prof. Liona POON, Chairperson of Department of Obstetrics and Gynaecology at CUHK’s Faculty of Medicine, examined molecular changes at maternal-fetal interface (MFI), where the mother’s body interacts with the developing fetus, following SARS-CoV-2 infection. Analyzing MFI samples from seven COVID-19 patients and seven healthy donors, the team discovered that SARS-CoV-2 infection triggers a significant increase in the immune response at the MFI. This heightened response is linked to the upregulation of interferon-related genes, which could expose the fetus to inflammation, hypoxia, and oxidative stress. Concurrently, the study identified dysregulation of angiogenesis genes at the MFI, potentially leading to abnormal blood vessel formation within the placenta and fetal growth restriction. Importantly, the observed molecular changes are likely due to the way the mother’s body responded to the virus when she first got infected, rather than the virus directly affecting the MFI tissues in the placenta.
The researchers also found that SARS-CoV-2 could affect placental development by impacting the regulation of pregnancy-related genes in specific placenta cells. The study identified that genetic elements called retrotransposons are dysregulated in placenta cell types, resulting in the downregulation of pregnancy-specific glycoprotein (PSG) genes. These genes play crucial roles in processes such as the placenta’s ability to develop blood vessels into the mother’s uterus, immune modulation in the placenta, and placental development, all of which can impact fetus health.
Prof. Leung said, “Our DNA serves as the blueprint for all biological processes. The precise activation and repression of genes are vital for normal growth and development. In diseases, genes are mis-regulated, causing errors in our normal physiological mechanisms. Therefore, in order to understand a disease and its impact on our cells, we must elucidate the alterations to gene expression patterns. Over the past two years, my laboratory and our collaborators have been dedicated to unraveling how SARS-CoV-2 influences gene expression and the subsequent consequences. Our findings contribute to a deeper understanding of the molecular mechanisms underlying COVID-19-related pregnancy complications without direct viral infection. This knowledge offers critical insights for future development of targeted interventions to mitigate the risk of adverse pregnancy outcomes associated with COVID-19 infection. Additionally, our work has provided insights into how epigenetic dysregulation of the PSG genes may contribute to pregnancy complications in other pregnancy-associated disorders, laying the groundwork for further studies on the role of PSG genes in other diseases and infections during pregnancy.”
Prof. Poon said, “During the pandemic, through epidemiological studies, we learned that pregnant patients with COVID-19 had increased risks for receiving critical care, mortality and pregnancy-specific morbidity including hypertensive disorders of pregnancy and embolic disease, and that infants born to women with COVID-19 were more likely to be preterm and have low birthweight. Now, our study has provided clues on how COVID-19 affects the placenta, thus causing a number of pregnancy complications. So our initial concerns regarding the negative impact of COVID-19 on maternal and perinatal outcome are real and therefore primary prevention is the key in order to reduce such risks. For women planning to get pregnant or who are pregnant, please get vaccinated timely. As we are coming out of the pandemic, the desire to get tested goes down, I would suggest that pregnant women with symptoms must get tested by the rapid antigen test and inform their healthcare providers so that relevant advice and antenatal follow up, including increased fetal growth surveillance, are provided.”
Their findings were published in the scientific journal Nature Cell Biology on July 3, 2023.
1 A meta-analysis led by the George Washington University represents 12 studies including 13,449 pregnant women from around the world. Information from 2,219 women with confirmed SARS-CoV-2 infection in pregnancy during Mar 2020-May 2021 was included.
2 According to a US study published in 2022, the prevalence of preterm birth in people with SARS-CoV-2 infection in pregnancy was higher (14%) relative to national baseline data (10.0–10.2%), particularly among people with third trimester infection.
3 From the data of Centers for Disease Control and Prevention on the maternal and infant characteristics among
women with confirmed or presumed cases of COVID-19 during pregnancy.
Journal
Nature Cell Biology
DOI
10.1038/s41556-023-01169-x
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Single-cell analysis reveals transcriptomic and epigenomic impacts on the maternal–fetal interface following SARS-CoV-2 infection
Article Publication Date
3-Jul-2023


